当前位置:
X-MOL 学术
›
Appl. Surf. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Study on the mechanism of ammonium carbamate in promoting the separation of chalcopyrite and arsenopyrite in oxidation systems
Applied Surface Science ( IF 6.3 ) Pub Date : 2024-12-17 , DOI: 10.1016/j.apsusc.2024.162127 Fan Wu, Dandan Wu, Qi Zuo, Jing Cao, Ning Kong, Kang Feng, Jianan Li, Shaojun Bai
Applied Surface Science ( IF 6.3 ) Pub Date : 2024-12-17 , DOI: 10.1016/j.apsusc.2024.162127 Fan Wu, Dandan Wu, Qi Zuo, Jing Cao, Ning Kong, Kang Feng, Jianan Li, Shaojun Bai
|
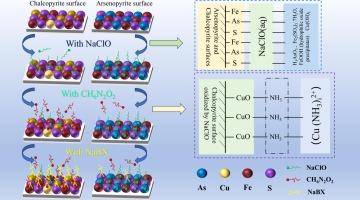
Arsenopyrite frequently occurs alongside chalcopyrite and is characterized by its high arsenic content. The similar surface properties of arsenopyrite and chalcopyrite pose significant challenges to their separation via flotation. This study investigated the inhibitory effect of sodium hypochlorite (NaClO) on arsenopyrite and the activation mechanism of ammonium carbamate on chalcopyrite through a series of single-mineral and artificial mixed-mineral flotation experiments. Techniques employed include atomic force microscopy (AFM), scanning electron microscopy (SEM) with energy-dispersive X-ray spectroscopy (EDS), X-ray photoelectron spectroscopy (XPS), Zeta potential analysis, and adsorption experiments. The results from flotation indicated that NaClO oxidation significantly suppressed the arsenopyrite recovery to as low as 7.80%, while chalcopyrite recovery was reduced to 54.02%. Upon the addition of ammonium carbamate, the flotation recovery of chalcopyrite increased to 81.71%, while the recovery of arsenopyrite remained largely unaffected. Further analysis with AFM, SEM, EDS, Zeta potential, adsorption tests, and XPS revealed that NaClO facilitated the formation of a hydrophilic film on the surface of arsenopyrite, reducing the adsorption of the trap on the minerals, and suppressing the hydrophobicity of arsenopyrite. Conversely, ammonium carbamate did not activate arsenopyrite but enhanced the adsorption of butyl xanthate on chalcopyrite. The results indicated that the combination of NaClO and ammonium carbamate presents an effective method for the selective separation of arsenopyrite and chalcopyrite.
中文翻译:

氨基甲酸铵促进氧化体系中黄铜矿与砷黄铁矿分离的机理研究
砷黄铁矿经常与黄铜矿一起出现,其特点是砷含量高。砷黄铁矿和黄铜矿的相似表面特性对它们通过浮选分离构成了重大挑战。本研究通过一系列单一矿物和人工混合矿物浮选实验,探讨了次氯酸钠 (NaClO) 对砷黄铁矿的抑制作用以及氨基甲酸铵对黄铜矿的活化机制。采用的技术包括原子力显微镜 (AFM)、扫描电子显微镜 (SEM) 与能量色散 X 射线光谱 (EDS)、X 射线光电子能谱 (XPS)、Zeta 电位分析和吸附实验。浮选结果表明,NaClO 氧化显著抑制了砷黄铁矿的回收率,使其低至 7.80%,而黄铜矿的回收率降低至 54.02%。添加氨基甲酸铵后,黄铜矿的浮选回收率提高到 81.71%,而砷黄铁矿的回收率基本不受影响。AFM、SEM、EDS、Zeta 电位、吸附试验和 XPS 的进一步分析表明,NaClO 促进了砷黄铁矿表面亲水膜的形成,减少了陷阱对矿物的吸附,并抑制了砷黄铁矿的疏水性。相反,氨基甲酸铵不活化砷黄铁矿,但增强了丁基黄原酸酯在黄铜矿上的吸附。结果表明,NaClO 与氨基甲酸铵的组合为选择性分离砷黄铁矿和黄铜矿提供了一种有效的方法。
更新日期:2024-12-20
中文翻译:

氨基甲酸铵促进氧化体系中黄铜矿与砷黄铁矿分离的机理研究
砷黄铁矿经常与黄铜矿一起出现,其特点是砷含量高。砷黄铁矿和黄铜矿的相似表面特性对它们通过浮选分离构成了重大挑战。本研究通过一系列单一矿物和人工混合矿物浮选实验,探讨了次氯酸钠 (NaClO) 对砷黄铁矿的抑制作用以及氨基甲酸铵对黄铜矿的活化机制。采用的技术包括原子力显微镜 (AFM)、扫描电子显微镜 (SEM) 与能量色散 X 射线光谱 (EDS)、X 射线光电子能谱 (XPS)、Zeta 电位分析和吸附实验。浮选结果表明,NaClO 氧化显著抑制了砷黄铁矿的回收率,使其低至 7.80%,而黄铜矿的回收率降低至 54.02%。添加氨基甲酸铵后,黄铜矿的浮选回收率提高到 81.71%,而砷黄铁矿的回收率基本不受影响。AFM、SEM、EDS、Zeta 电位、吸附试验和 XPS 的进一步分析表明,NaClO 促进了砷黄铁矿表面亲水膜的形成,减少了陷阱对矿物的吸附,并抑制了砷黄铁矿的疏水性。相反,氨基甲酸铵不活化砷黄铁矿,但增强了丁基黄原酸酯在黄铜矿上的吸附。结果表明,NaClO 与氨基甲酸铵的组合为选择性分离砷黄铁矿和黄铜矿提供了一种有效的方法。






























 京公网安备 11010802027423号
京公网安备 11010802027423号