当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Orvinol-based opioid receptor antagonist fluorinated at C(20)-pharmacophore
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2024-12-18 , DOI: 10.1016/j.ejmech.2024.117189 A.A. Ambartsumyan, I.V. Belozertseva, O.А. Dravolina, E.E. Zvartau, I.V. Sandulenko, M.V. Zelentsova, A.S. Peregudov, S.K. Moiseev
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2024-12-18 , DOI: 10.1016/j.ejmech.2024.117189 A.A. Ambartsumyan, I.V. Belozertseva, O.А. Dravolina, E.E. Zvartau, I.V. Sandulenko, M.V. Zelentsova, A.S. Peregudov, S.K. Moiseev
|
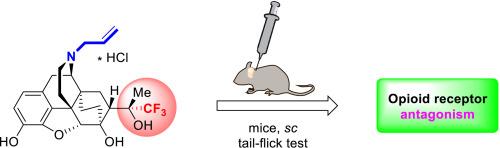
Thevinols and their 3-O-demethylated relatives, orvinols, are derivatives of the Diels-Alder adduct of natural alkaloid thebaine with methyl vinyl ketone. Taken together, thevinols and orvinols constitute an important family of opioid receptor (OR) ligands playing an important role in both the OR mediated antinociception and OR antagonism. Herein, we disclose for the first time the antagonist activity of the N-allyl substituted orvinol derivative fluorinated within the pharmacophore associated with C(20) and its surrounding. This compound was prepared via a novel synthetic sequence from 18,19-dihydrothevinone bearing an allyl substituent at N(17) and three fluorine atoms at C(21). Preliminary trials reported earlier demonstrated that the compound exhibited no analgesic activity. However, in vivo experiments conducted in an acute pain model (tail-flick test in mice) demonstrated that this fluorinated compound, when administered at doses of 5-10 mg/kg (sc) 30 min before morphine, exhibited antagonistic activity at the level of naloxone (1 mg/kg, sc) for a longer duration (at least 120 min) compared to naloxone (60 min). Together with the analgesic activity that has been reported for the C(21)-trifluorinated relatives bearing methyl or cyclopropylmethyl substituent at N(17), this result highlights C(21)-fluorinated thevinols and orvinols as the family of opioid receptor ligands (structurally related to buprenorphine, diprenorphine, etc.) covering the full range of activity profiles from agonists to antagonists, which is promising for tuning of their pharmacological properties via a substitution of hydrogen atoms within the pharmacophore associated with C(20) and its surrounding for fluorine.
中文翻译:

基于 Orvinol 的阿片受体拮抗剂,在 C(20) -药效团中氟化
Thevinols 及其 3-O-去甲基化亲戟 orvinols 是天然生物碱 thebaine 与甲基乙烯基酮的 Diels-Alder 加合物的衍生物。综上所述,thevinols 和 orvinols 构成了阿片受体 (OR) 配体的重要家族,在 OR 介导的抗伤害感受和 OR 拮抗作用中起重要作用。在此,我们首次公开了与 C(20) 及其周围相关的药效团内氟化的 N-烯丙基取代的 orvinol 衍生物的拮抗剂活性。该化合物是通过 18,19-二氢色乙烯酮的新型合成序列制备的,该化合物在 N(17) 处带有烯丙基取代基,在 C(21) 处带有三个氟原子。早些时候报告的初步试验表明,该化合物没有表现出镇痛活性。然而,在急性疼痛模型(小鼠甩尾巴测试)中进行的体内实验表明,当吗啡前 30 分钟以 5-10 mg/kg (sc) 的剂量施用这种氟化化合物时,在纳洛酮水平(1 mg/kg,sc)表现出拮抗活性持续时间更长(至少 120 分钟)与纳洛酮(60 分钟)。结合已报道的在 N(17) 处带有甲基或环丙基甲基取代基的 C(21)-三氟亲戚的镇痛活性,该结果突出了 C(21)-氟化酪醇和奥维诺醇作为阿片受体配体家族(结构上与丁丙诺啡、二丙诺啡等相关),涵盖了从激动剂到拮抗剂的所有活性曲线, 这很有希望通过与 C(20) 相关的药效团内的氢原子替换为氟来调节它们的药理学特性。
更新日期:2024-12-18
中文翻译:

基于 Orvinol 的阿片受体拮抗剂,在 C(20) -药效团中氟化
Thevinols 及其 3-O-去甲基化亲戟 orvinols 是天然生物碱 thebaine 与甲基乙烯基酮的 Diels-Alder 加合物的衍生物。综上所述,thevinols 和 orvinols 构成了阿片受体 (OR) 配体的重要家族,在 OR 介导的抗伤害感受和 OR 拮抗作用中起重要作用。在此,我们首次公开了与 C(20) 及其周围相关的药效团内氟化的 N-烯丙基取代的 orvinol 衍生物的拮抗剂活性。该化合物是通过 18,19-二氢色乙烯酮的新型合成序列制备的,该化合物在 N(17) 处带有烯丙基取代基,在 C(21) 处带有三个氟原子。早些时候报告的初步试验表明,该化合物没有表现出镇痛活性。然而,在急性疼痛模型(小鼠甩尾巴测试)中进行的体内实验表明,当吗啡前 30 分钟以 5-10 mg/kg (sc) 的剂量施用这种氟化化合物时,在纳洛酮水平(1 mg/kg,sc)表现出拮抗活性持续时间更长(至少 120 分钟)与纳洛酮(60 分钟)。结合已报道的在 N(17) 处带有甲基或环丙基甲基取代基的 C(21)-三氟亲戚的镇痛活性,该结果突出了 C(21)-氟化酪醇和奥维诺醇作为阿片受体配体家族(结构上与丁丙诺啡、二丙诺啡等相关),涵盖了从激动剂到拮抗剂的所有活性曲线, 这很有希望通过与 C(20) 相关的药效团内的氢原子替换为氟来调节它们的药理学特性。






























 京公网安备 11010802027423号
京公网安备 11010802027423号