当前位置:
X-MOL 学术
›
Nano Energy
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
High hydrophilic/zincophilic interpenetrating double-network hydrogel electrolyte constructing stable organic-inorganic anode interface toward nickel–zinc batteries
Nano Energy ( IF 16.8 ) Pub Date : 2024-12-17 , DOI: 10.1016/j.nanoen.2024.110595 Hongyan Yuan, Jingyi Luan, Quanchao Zhang, Jie Liu, Naiqin Zhao, Wenbin Hu, Cheng Zhong
Nano Energy ( IF 16.8 ) Pub Date : 2024-12-17 , DOI: 10.1016/j.nanoen.2024.110595 Hongyan Yuan, Jingyi Luan, Quanchao Zhang, Jie Liu, Naiqin Zhao, Wenbin Hu, Cheng Zhong
|
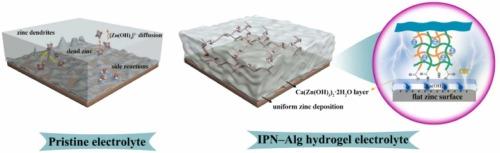
Nickel–zinc batteries are attracting growing interest due to flame-retardant properties, high discharge voltage and attractive power density. However, the interface side reactions, dendrite growth and redistribution of the highly soluble [Zn(OH)4]2− on the electrode surface result in the degradation of the zinc anode. Herein, an interpenetrating polymer network hydrogel (denoted as IPN–Alg) is prepared by introducing alginate and a stable organic–inorganic interface is successfully constructed in situ on the zinc anode. The high hydrophilicity and zincophilicity of IPN–Alg hydrogel electrolyte provide the inherent advantages in reducing the amounts of free water to suppress the side reactions and being preferentially adsorbed on the zinc anode to construct a water-poor interface. Moreover, due to the topological entanglement in the interpenetrating structures, the IPN–Alg hydrogel electrolyte exhibits excellent mechanical strength. Combining with the in situ formation of the inorganic protective layer of Ca(Zn(OH)3)2·2H2O, the robust organic–inorganic interface layer can effectively inhibit the dendrite growth and reduce the diffusion and redistribution of [Zn(OH)4]2−. Hence, the Zn||Zn symmetric cell and nickel–zinc pouch battery based on IPN–Alg hydrogel electrolyte demonstrate ultralong cycling life of more than 800 h at 2 mA cm−2 and 1100 h (563 cycles) at 4 C, 40% DOD (depth of discharge), respectively.
中文翻译:

高亲水/亲锌互穿双网络水凝胶电解质构建面向镍锌电池的稳定有机-无机负极界面
镍锌电池因其阻燃性能、高放电电压和有吸引力的功率密度而引起了越来越多的关注。然而,界面副反应、枝晶生长和电极表面高度可溶性 [Zn(OH)4]2− 的重新分布导致锌阳极降解。在此,通过引入海藻酸盐制备了互穿聚合物网络水凝胶(表示为 IPN-Alg),并在锌阳极上成功原位构建了稳定的有机-无机界面。IPN-Alg 水凝胶电解质的高亲水性和亲锌性提供了减少游离水量以抑制副反应并优先被锌阳极吸收以构建缺水界面的固有优势。此外,由于互穿结构中的拓扑纠缠,IPN-Alg 水凝胶电解质表现出优异的机械强度。结合 Ca(Zn(OH)3)2·2H2O 的原位形成,坚固的有机-无机界面层可以有效抑制枝晶生长并减少 [Zn(OH)4]2− 的扩散和重新分布。因此,Zn||基于 IPN-Alg 水凝胶电解质的 Zn 对称电池和镍锌软包电池在 2mAcm-2 和 4C、40% DOD(放电深度)下分别表现出超过 800h 的超长循环寿命超过 800h 和 1100h(563 次循环)。
更新日期:2024-12-21
中文翻译:

高亲水/亲锌互穿双网络水凝胶电解质构建面向镍锌电池的稳定有机-无机负极界面
镍锌电池因其阻燃性能、高放电电压和有吸引力的功率密度而引起了越来越多的关注。然而,界面副反应、枝晶生长和电极表面高度可溶性 [Zn(OH)4]2− 的重新分布导致锌阳极降解。在此,通过引入海藻酸盐制备了互穿聚合物网络水凝胶(表示为 IPN-Alg),并在锌阳极上成功原位构建了稳定的有机-无机界面。IPN-Alg 水凝胶电解质的高亲水性和亲锌性提供了减少游离水量以抑制副反应并优先被锌阳极吸收以构建缺水界面的固有优势。此外,由于互穿结构中的拓扑纠缠,IPN-Alg 水凝胶电解质表现出优异的机械强度。结合 Ca(Zn(OH)3)2·2H2O 的原位形成,坚固的有机-无机界面层可以有效抑制枝晶生长并减少 [Zn(OH)4]2− 的扩散和重新分布。因此,Zn||基于 IPN-Alg 水凝胶电解质的 Zn 对称电池和镍锌软包电池在 2mAcm-2 和 4C、40% DOD(放电深度)下分别表现出超过 800h 的超长循环寿命超过 800h 和 1100h(563 次循环)。






























 京公网安备 11010802027423号
京公网安备 11010802027423号