当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Combined Kinetic and Computational Analysis of the Palladium-Catalyzed Formylation of Aryl Bromides
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-12-18 , DOI: 10.1021/acscatal.4c05324 Georgina Rai, Lee J. Edwards, Rebecca L. Greenaway, Philip W. Miller, Katherine M. P. Wheelhouse, Mark R. Crimmin
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-12-18 , DOI: 10.1021/acscatal.4c05324 Georgina Rai, Lee J. Edwards, Rebecca L. Greenaway, Philip W. Miller, Katherine M. P. Wheelhouse, Mark R. Crimmin
|
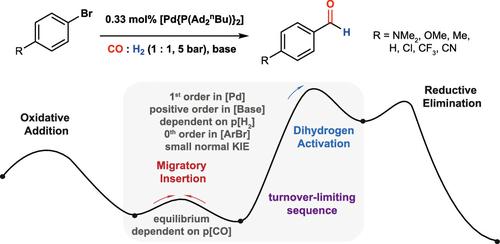
Aryl aldehydes are key synthetic intermediates in the manufacturing of active pharmaceutical ingredients. They are generated on scale (>1000 kg) through the palladium-catalyzed formylation of aryl bromides using syngas (CO/H2). The best-in-class catalyst system for this reaction employs di-1-adamantyl-n-butylphosphine (cataCXium A), palladium(II) acetate, and tetramethylethylenediamine. Despite nearly 20 years since its initial report, a mechanistic understanding of this system remains incomplete. Here, we use automation, kinetic analysis, and DFT calculations to develop a mechanistic model for this best-in-class catalyst. We suggest that a combination of the migratory insertion step and dihydrogen activation step is likely involved in the turnover-limiting sequence. The reaction kinetics are responsive to the nature of the substrate, with electron-rich aryl bromides reacting faster and more selectively than their electron-poor counterparts due to the influence of electronics in the migratory insertion step. Our findings add additional insight into the proposed mechanism of palladium-catalyzed formylation of aryl bromides.
中文翻译:

钯催化的芳基溴甲酰化的动力学和计算相结合分析
芳基醛是制造活性药物成分的关键合成中间体。它们是通过使用合成气 (CO/H2) 对芳基溴进行钯催化的甲酰化反应成比例 (>1000 kg) 生成的。该反应的一流催化剂系统采用二-1-金刚烷基-正丁基膦 (cataCXium A)、乙酸钯 (II) 和四甲基乙二胺。尽管自其首次报告以来已经过去了近 20 年,但对该系统的机械理解仍然不完整。在这里,我们使用自动化、动力学分析和 DFT 计算来为这种一流的催化剂开发机理模型。我们认为迁移插入步骤和二氢活化步骤的组合可能涉及周转限制序列。反应动力学对底物的性质有反应,由于迁移插入步骤中电子元件的影响,富电子芳基溴的反应比贫电子芳基溴反应更快、选择性更强。我们的研究结果为钯催化芳基溴的甲酰化机制增加了额外的见解。
更新日期:2024-12-18
中文翻译:

钯催化的芳基溴甲酰化的动力学和计算相结合分析
芳基醛是制造活性药物成分的关键合成中间体。它们是通过使用合成气 (CO/H2) 对芳基溴进行钯催化的甲酰化反应成比例 (>1000 kg) 生成的。该反应的一流催化剂系统采用二-1-金刚烷基-正丁基膦 (cataCXium A)、乙酸钯 (II) 和四甲基乙二胺。尽管自其首次报告以来已经过去了近 20 年,但对该系统的机械理解仍然不完整。在这里,我们使用自动化、动力学分析和 DFT 计算来为这种一流的催化剂开发机理模型。我们认为迁移插入步骤和二氢活化步骤的组合可能涉及周转限制序列。反应动力学对底物的性质有反应,由于迁移插入步骤中电子元件的影响,富电子芳基溴的反应比贫电子芳基溴反应更快、选择性更强。我们的研究结果为钯催化芳基溴的甲酰化机制增加了额外的见解。






























 京公网安备 11010802027423号
京公网安备 11010802027423号