当前位置:
X-MOL 学术
›
Electrochim. Acta
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Description of ions properties using molecular orbital energy levels: Trends in interaction with model electrodes and solvents
Electrochimica Acta ( IF 5.5 ) Pub Date : 2024-12-17 , DOI: 10.1016/j.electacta.2024.145541 Sergey V. Doronin
Electrochimica Acta ( IF 5.5 ) Pub Date : 2024-12-17 , DOI: 10.1016/j.electacta.2024.145541 Sergey V. Doronin
|
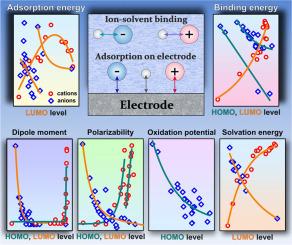
The study reveals correlations between the parameters of ions and their HOMO and LUMO orbital energy level values. In particular, it demonstrates a clear correlation for the ion adsorption parameters on model electrodes: the aluminum oxide (0001) surface, graphene and Au (111) surface. Correlations are also observed for the parameters of ion binding to water and dimethyl carbonate molecules, which are often used as solvents. In addition, the dipole moment, polarizability and solvation energy of ions are well correlated with the values of the molecular orbital energies, and for anions a dependence on the oxidation potential is observed. The experimental ion parameters reported in the literature also show correlations with the energy levels of the ion orbitals. The obtained descriptors make it possible to select ions with desired values for a specific problem. As an illustrative example, in this work we consider the problem of displacement of water molecules from the inner electric double layer by ions, which is one of the factors increasing the potential window in electrolytes of aqueous batteries. This approach can be applied in the rapidly developing field of aqueous electrolytes for battery or supercapacitor design, catalysis control through surface composition variations, as well as in studies of heavy metal ion binding to sorption materials.
中文翻译:

使用分子轨道能级描述离子性质:与模型电极和溶剂相互作用的趋势
该研究揭示了离子参数与其 HOMO 和 LUMO 轨道能级值之间的相关性。特别是,它证明了模型电极上的离子吸附参数的明显相关性:氧化铝 (0001) 表面、石墨烯和金 (111) 表面。还观察到离子与水和碳酸二甲酯分子(通常用作溶剂)的结合参数的相关性。此外,离子的偶极矩、极化率和溶剂化能与分子轨道能量的值密切相关,对于阴离子,观察到对氧化电位的依赖性。文献中报道的实验离子参数也显示出与离子轨道能级的相关性。获得的描述符可以为特定问题选择具有所需值的离子。作为一个说明性的例子,在这项工作中,我们考虑了水分子被离子从内电双电层位移的问题,这是增加水系电池电解质中电位窗口的因素之一。这种方法可应用于快速发展的水性电解质领域,用于电池或超级电容器设计,通过表面成分变化进行催化控制,以及重金属离子与吸附材料结合的研究。
更新日期:2024-12-17
中文翻译:

使用分子轨道能级描述离子性质:与模型电极和溶剂相互作用的趋势
该研究揭示了离子参数与其 HOMO 和 LUMO 轨道能级值之间的相关性。特别是,它证明了模型电极上的离子吸附参数的明显相关性:氧化铝 (0001) 表面、石墨烯和金 (111) 表面。还观察到离子与水和碳酸二甲酯分子(通常用作溶剂)的结合参数的相关性。此外,离子的偶极矩、极化率和溶剂化能与分子轨道能量的值密切相关,对于阴离子,观察到对氧化电位的依赖性。文献中报道的实验离子参数也显示出与离子轨道能级的相关性。获得的描述符可以为特定问题选择具有所需值的离子。作为一个说明性的例子,在这项工作中,我们考虑了水分子被离子从内电双电层位移的问题,这是增加水系电池电解质中电位窗口的因素之一。这种方法可应用于快速发展的水性电解质领域,用于电池或超级电容器设计,通过表面成分变化进行催化控制,以及重金属离子与吸附材料结合的研究。






























 京公网安备 11010802027423号
京公网安备 11010802027423号