当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iridium-Catalyzed Enantioconvergent Construction of Piperidines and Tetrahydroisoquinolines from Racemic 1,5-Diols
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-12-17 , DOI: 10.1021/jacs.4c12466 Huanlin Diao, Kexin Liu, Rong Yu, Jilin Chen, Yongbing Liu, Bin-Miao Yang, Yu Zhao
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-12-17 , DOI: 10.1021/jacs.4c12466 Huanlin Diao, Kexin Liu, Rong Yu, Jilin Chen, Yongbing Liu, Bin-Miao Yang, Yu Zhao
|
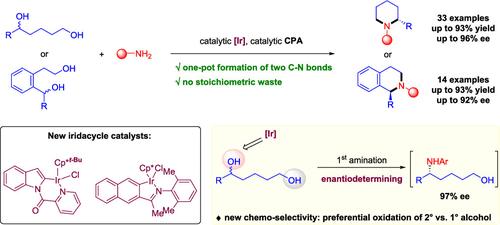
We report herein a one-step synthesis of valuable enantioenriched piperidines and tetrahydroisoquinolines from readily available racemic 1,5-diols. Key to the success is the development of new iridacycle catalysts that enable efficient redox-neutral construction of two C–N bonds between diols and amines in an enantioconvergent fashion. Mechanistic studies identified an intriguing preferential oxidation of secondary versus primary alcohol in the diol substrate by the iridacycle catalyst, which set a challenging intermolecular amination of aryl–alkyl-substituted alcohol as the enantiodetermining step for this catalytic N-heterocycle synthesis. Application of this catalytic method to the preparation of important drugs and bioactive compounds is also demonstrated.
中文翻译:

铱催化的外消旋 1,5-二醇对映体 piperidines 和四氢异喹啉的对映趋同构建
我们在此报道了从现成的外消旋 1,5-二醇中合成有价值的富含对映体的哌啶和四氢异喹啉的一步法。成功的关键是开发新的虹膜循环催化剂,能够以对映收敛方式在二醇和胺之间高效构建两个 C-N 键的氧化还原中性结构。机理研究确定了虹膜环催化剂在二醇底物中仲醇与伯醇之间的有趣优先氧化,该催化剂将芳基-烷基取代醇的具有挑战性的分子间胺化设置为该催化 N-杂环合成的对映决定步骤。还证明了这种催化方法在重要药物和生物活性化合物制备中的应用。
更新日期:2024-12-17
中文翻译:

铱催化的外消旋 1,5-二醇对映体 piperidines 和四氢异喹啉的对映趋同构建
我们在此报道了从现成的外消旋 1,5-二醇中合成有价值的富含对映体的哌啶和四氢异喹啉的一步法。成功的关键是开发新的虹膜循环催化剂,能够以对映收敛方式在二醇和胺之间高效构建两个 C-N 键的氧化还原中性结构。机理研究确定了虹膜环催化剂在二醇底物中仲醇与伯醇之间的有趣优先氧化,该催化剂将芳基-烷基取代醇的具有挑战性的分子间胺化设置为该催化 N-杂环合成的对映决定步骤。还证明了这种催化方法在重要药物和生物活性化合物制备中的应用。






























 京公网安备 11010802027423号
京公网安备 11010802027423号