当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Switching CO-to-Acetate Electroreduction on Cu Atomic Ensembles
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-12-17 , DOI: 10.1021/jacs.4c13197 Libing Zhang, Jiaqi Feng, Ruhan Wang, Limin Wu, Xinning Song, Xiangyuan Jin, Xingxing Tan, Shunhan Jia, Xiaodong Ma, Lihong Jing, Qinggong Zhu, Xinchen Kang, Jianling Zhang, Xiaofu Sun, Buxing Han
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-12-17 , DOI: 10.1021/jacs.4c13197 Libing Zhang, Jiaqi Feng, Ruhan Wang, Limin Wu, Xinning Song, Xiangyuan Jin, Xingxing Tan, Shunhan Jia, Xiaodong Ma, Lihong Jing, Qinggong Zhu, Xinchen Kang, Jianling Zhang, Xiaofu Sun, Buxing Han
|
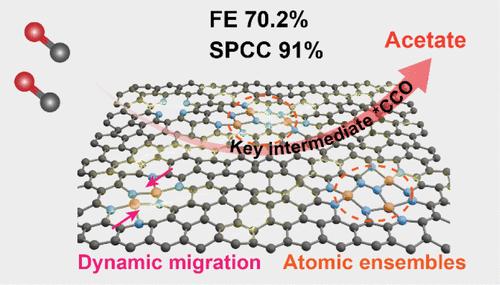
The electrocatalytic reaction pathway is highly dependent on the intrinsic structure of the catalyst. CO2/CO electroreduction has recently emerged as a potential approach for obtaining C2+ products, but it is challenging to achieve high selectivity for a single C2+ product. Herein, we develop a Cu atomic ensemble that satisfies the appropriate site distance and coordination environment required for electrocatalytic CO-to-acetate conversion, which shows outstanding overall performance with an acetate Faradaic efficiency of 70.2% with a partial current density of 225 mA cm–2 and a formation rate of 2.1 mmol h–1 cm–2. Moreover, a single-pass CO conversion rate of 91% and remarkable stability can be also obtained. Detailed experimental and theoretical investigations confirm the significant advantages of the Cu atomic ensembles in optimizing C–C coupling, stabilizing key ketene intermediate (*CCO), and inhibiting the *HOCCOH intermediate, which can switch the CO reduction pathway from the ethanol/ethylene on the conventional metallic Cu site to the acetate on the Cu atomic ensembles.
中文翻译:

在 Cu 原子系综上切换 CO 到乙酸盐电还原
电催化反应途径高度依赖于催化剂的本征结构。CO2/CO 电还原最近已成为获得 C2+ 产物的一种潜在方法,但要实现单个 C2+ 产物的高选择性具有挑战性。在此,我们开发了一种 Cu 原子系综,它满足电催化 CO 到乙酸盐转化所需的适当位距和配位环境,它显示出出色的整体性能,乙酸盐法拉第效率为 70.2%,部分电流密度为 225 mA cm–2,形成速率为 2.1 mmol h–1 cm–2.此外,还可以获得 91% 的单程 CO 转化率和显着的稳定性。详细的实验和理论研究证实了 Cu 原子系合体在优化 C-C 偶联、稳定关键乙烯酮中间体 (*CCO) 和抑制 *HOCCOH 中间体方面的显着优势,它可以将 CO 还原途径从常规金属 Cu 位点上的乙醇/乙烯切换到 Cu 原子系综上的乙酸盐。
更新日期:2024-12-17
中文翻译:

在 Cu 原子系综上切换 CO 到乙酸盐电还原
电催化反应途径高度依赖于催化剂的本征结构。CO2/CO 电还原最近已成为获得 C2+ 产物的一种潜在方法,但要实现单个 C2+ 产物的高选择性具有挑战性。在此,我们开发了一种 Cu 原子系综,它满足电催化 CO 到乙酸盐转化所需的适当位距和配位环境,它显示出出色的整体性能,乙酸盐法拉第效率为 70.2%,部分电流密度为 225 mA cm–2,形成速率为 2.1 mmol h–1 cm–2.此外,还可以获得 91% 的单程 CO 转化率和显着的稳定性。详细的实验和理论研究证实了 Cu 原子系合体在优化 C-C 偶联、稳定关键乙烯酮中间体 (*CCO) 和抑制 *HOCCOH 中间体方面的显着优势,它可以将 CO 还原途径从常规金属 Cu 位点上的乙醇/乙烯切换到 Cu 原子系综上的乙酸盐。






























 京公网安备 11010802027423号
京公网安备 11010802027423号