当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Borenium-Catalyzed “Boron Walking” for Remote Site-Selective Hydroboration
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-12-17 , DOI: 10.1021/jacs.4c13726 Zheng Zhu, Wing Chun Chan, Bin Gao, Guanwen Hu, Peiqi Zhang, Yiyi Fu, Kit San Ly, Zhenyang Lin, Yangjian Quan
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-12-17 , DOI: 10.1021/jacs.4c13726 Zheng Zhu, Wing Chun Chan, Bin Gao, Guanwen Hu, Peiqi Zhang, Yiyi Fu, Kit San Ly, Zhenyang Lin, Yangjian Quan
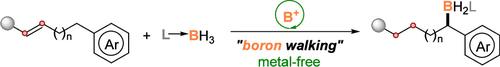
|
Remote functionalization through progressive olefin isomerization enables site-selective modification at a distal position, diversifying the synthetic approaches. However, the developed protocols have long relied on transition metal catalysis. Transition metal catalysts are deemed irreplaceable, albeit facing challenges in metal residue and catalyst poisoning. In this work, we present a pioneering approach that employs a borenium ion as a catalyst for site-selective, remote borylation, eliminating the need for metal catalysts. As the reaction progresses, borylation isomers at different positions emerge, gradually and ultimately converging into the predominant α-borylation product. This process is akin to a “walking” of a boron moiety along a carbon skeleton toward an aryl terminus. Detailed mechanistic studies and DFT calculations substantiate the borenium-catalyzed, stepwise migration via a reversible B–H insertion/elimination sequence. This remote borylation exhibits good functional group compatibility, complementing those methods reliant on transition metals. Furthermore, this metal-free protocol permits the convenient synthesis of silyl-remote-boryl compounds, demonstrating an opposite regioselectivity to that observed in transition-metal-catalyzed tandem silylation-borylation reactions. This discovery therefore contributes to site-selective, remote difunctionalization via sequential C–B and C–Si derivatizations, exemplified by the synthesis of amino-remote-alcohol bioactive molecules.
中文翻译:

硼催化的“硼行走”用于远程位点选择性硼氢化
通过渐进式烯烃异构化实现远程官能化,可在远端位置进行位点选择性修饰,使合成方法多样化。然而,开发的方案长期以来一直依赖于过渡金属催化。过渡金属催化剂被认为是不可替代的,尽管面临金属残留和催化剂中毒的挑战。在这项工作中,我们提出了一种开创性的方法,该方法采用硼离子作为催化剂进行位点选择性、远程硼酸化,无需金属催化剂。随着反应的进行,不同位置的硼酸化异构体出现,逐渐并最终收敛到主要的 α-硼酸化产物中。这个过程类似于硼部分沿着碳骨架向芳基末端“行走”。详细的机理研究和 DFT 计算证实了硼催化的通过可逆 B-H 插入/消除序列的逐步迁移。这种远程硼酸化表现出良好的官能团相容性,补充了那些依赖于过渡金属的方法。此外,这种无金属方案允许方便地合成硅烷基远程硼基化合物,表现出与过渡金属催化的串联硅烷基化-硼化反应中观察到的相反的区域选择性。因此,这一发现有助于通过连续的 C-B 和 C-Si 衍生化进行位点选择性、远程二官能化,以氨基-远程醇生物活性分子的合成为例。
更新日期:2024-12-17
中文翻译:

硼催化的“硼行走”用于远程位点选择性硼氢化
通过渐进式烯烃异构化实现远程官能化,可在远端位置进行位点选择性修饰,使合成方法多样化。然而,开发的方案长期以来一直依赖于过渡金属催化。过渡金属催化剂被认为是不可替代的,尽管面临金属残留和催化剂中毒的挑战。在这项工作中,我们提出了一种开创性的方法,该方法采用硼离子作为催化剂进行位点选择性、远程硼酸化,无需金属催化剂。随着反应的进行,不同位置的硼酸化异构体出现,逐渐并最终收敛到主要的 α-硼酸化产物中。这个过程类似于硼部分沿着碳骨架向芳基末端“行走”。详细的机理研究和 DFT 计算证实了硼催化的通过可逆 B-H 插入/消除序列的逐步迁移。这种远程硼酸化表现出良好的官能团相容性,补充了那些依赖于过渡金属的方法。此外,这种无金属方案允许方便地合成硅烷基远程硼基化合物,表现出与过渡金属催化的串联硅烷基化-硼化反应中观察到的相反的区域选择性。因此,这一发现有助于通过连续的 C-B 和 C-Si 衍生化进行位点选择性、远程二官能化,以氨基-远程醇生物活性分子的合成为例。






























 京公网安备 11010802027423号
京公网安备 11010802027423号