Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
YTHDF2 promotes ATP synthesis and immune evasion in B cell malignancies
Cell ( IF 45.5 ) Pub Date : 2024-12-17 , DOI: 10.1016/j.cell.2024.11.007
Zhenhua Chen 1 , Chengwu Zeng 2 , Lu Yang 1 , Yuan Che 1 , Meiling Chen 3 , Lillian Sau 1 , Bintao Wang 1 , Keren Zhou 1 , Yu Chen 4 , Ying Qing 1 , Chao Shen 1 , Tingjian Zhang 5 , Mark Wunderlich 6 , Dong Wu 1 , Wei Li 1 , Kitty Wang 1 , Keith Leung 1 , Miao Sun 7 , Tingting Tang 1 , Xin He 8 , Lianjun Zhang 8 , Srividya Swaminathan 9 , James C Mulloy 6 , Markus Müschen 10 , Huilin Huang 11 , Hengyou Weng 12 , Gang Xiao 13 , Xiaolan Deng 1 , Jianjun Chen 1
Cell ( IF 45.5 ) Pub Date : 2024-12-17 , DOI: 10.1016/j.cell.2024.11.007
Zhenhua Chen 1 , Chengwu Zeng 2 , Lu Yang 1 , Yuan Che 1 , Meiling Chen 3 , Lillian Sau 1 , Bintao Wang 1 , Keren Zhou 1 , Yu Chen 4 , Ying Qing 1 , Chao Shen 1 , Tingjian Zhang 5 , Mark Wunderlich 6 , Dong Wu 1 , Wei Li 1 , Kitty Wang 1 , Keith Leung 1 , Miao Sun 7 , Tingting Tang 1 , Xin He 8 , Lianjun Zhang 8 , Srividya Swaminathan 9 , James C Mulloy 6 , Markus Müschen 10 , Huilin Huang 11 , Hengyou Weng 12 , Gang Xiao 13 , Xiaolan Deng 1 , Jianjun Chen 1
Affiliation
|
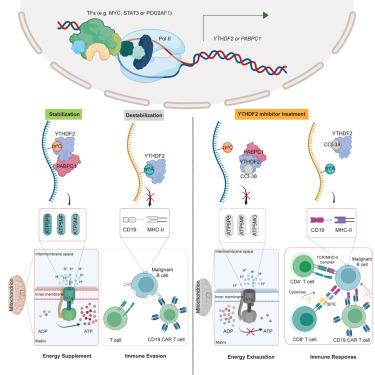
Long-term durable remission in patients with B cell malignancies following chimeric antigen receptor (CAR)-T cell immunotherapy remains unsatisfactory, often due to antigen escape. Malignant B cell transformation and oncogenic growth relies on efficient ATP synthesis, although the underlying mechanisms remain unclear. Here, we report that YTHDF2 facilitates energy supply and antigen escape in B cell malignancies, and its overexpression alone is sufficient to cause B cell transformation and tumorigenesis. Mechanistically, YTHDF2 functions as a dual reader where it stabilizes mRNAs as a 5-methylcytosine (m5 C) reader via recruiting PABPC1, thereby enhancing their expression and ATP synthesis. Concomitantly, YTHDF2 also promotes immune evasion by destabilizing other mRNAs as an N 6 -methyladenosine (m6 A) reader. Small-molecule-mediated targeting of YTHDF2 suppresses aggressive B cell malignancies and sensitizes them to CAR-T cell therapy.
中文翻译:

YTHDF2 促进 B 细胞恶性肿瘤中的 ATP 合成和免疫逃逸
嵌合抗原受体 (CAR)-T 细胞免疫治疗后 B 细胞恶性肿瘤患者的长期持久缓解仍然不令人满意,通常是由于抗原逃逸。恶性 B 细胞转化和致癌生长依赖于有效的 ATP 合成,但其潜在机制尚不清楚。在这里,我们报道了 YTHDF2 促进 B 细胞恶性肿瘤中的能量供应和抗原逃逸,仅其过表达就足以引起 B 细胞转化和肿瘤发生。从机制上讲,YTHDF2 起双读取器的作用,它通过募集 PABPC1 将 mRNA 稳定为 5-甲基胞嘧啶 (m5C) 读取器,从而增强它们的表达和 ATP 合成。同时,YTHDF2 还通过破坏其他 mRNA 作为 N6-甲基腺苷 (m6A) 读取器的稳定性来促进免疫逃避。YTHDF2 的小分子介导靶向抑制侵袭性 B 细胞恶性肿瘤,并使它们对 CAR-T 细胞疗法敏感。
更新日期:2024-12-17
中文翻译:

YTHDF2 促进 B 细胞恶性肿瘤中的 ATP 合成和免疫逃逸
嵌合抗原受体 (CAR)-T 细胞免疫治疗后 B 细胞恶性肿瘤患者的长期持久缓解仍然不令人满意,通常是由于抗原逃逸。恶性 B 细胞转化和致癌生长依赖于有效的 ATP 合成,但其潜在机制尚不清楚。在这里,我们报道了 YTHDF2 促进 B 细胞恶性肿瘤中的能量供应和抗原逃逸,仅其过表达就足以引起 B 细胞转化和肿瘤发生。从机制上讲,YTHDF2 起双读取器的作用,它通过募集 PABPC1 将 mRNA 稳定为 5-甲基胞嘧啶 (m5C) 读取器,从而增强它们的表达和 ATP 合成。同时,YTHDF2 还通过破坏其他 mRNA 作为 N6-甲基腺苷 (m6A) 读取器的稳定性来促进免疫逃避。YTHDF2 的小分子介导靶向抑制侵袭性 B 细胞恶性肿瘤,并使它们对 CAR-T 细胞疗法敏感。

































 京公网安备 11010802027423号
京公网安备 11010802027423号