当前位置:
X-MOL 学术
›
Adv. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Mitochondrion‐Targeting Piezoelectric Nanosystem for the Treatment of Erectile Dysfunction via Autophagy Regulation
Advanced Materials ( IF 27.4 ) Pub Date : 2024-12-17 , DOI: 10.1002/adma.202413287 Shuting Wang, Zhenqing Wang, Zhenjie Zang, Xiaojie Liang, Bin Jia, Tan Ye, Yang Lan, Xuetao Shi
Advanced Materials ( IF 27.4 ) Pub Date : 2024-12-17 , DOI: 10.1002/adma.202413287 Shuting Wang, Zhenqing Wang, Zhenjie Zang, Xiaojie Liang, Bin Jia, Tan Ye, Yang Lan, Xuetao Shi
|
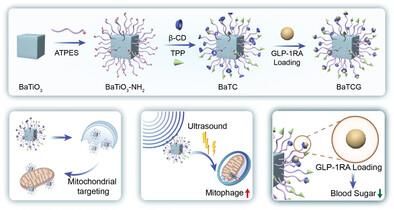
Mitochondrial damage caused by external stimuli, such as high glucose levels and inflammation, results in excessive reactive oxygen species (ROS) production. Existing antioxidants can only scavenge ROS and cannot address the root cause of ROS production, namely, abnormal mitochondria. To overcome this limitation, the study develops a piezoelectric synergistic drug‐loaded nanosystem (BaTCG nanosystem) that targets mitochondria. The BaTCG nanosystem is delivered to mitochondria via triphenylphosphine modification, and generates current under the stimulation of ultrasound, thereby promoting mitochondrial autophagy and restoring mitochondrial homeostasis. In a model of diabetes‐related erectile dysfunction (ED), the BaTCG nanosystem, through the current induced by the piezoelectric effect, not only promoted mitophagy, thereby reducing ROS production, but also released long‐acting glucagon‐like peptide‐1 receptor agonists (GLP‐1RAs) to effectively reduce blood glucose levels and mitochondrial damage. Each component of this nanosystem functions individually as well as synergistically, thus facilitating corpus cavernosum repair and restoring erectile function. In conclusion, the findings offer a novel therapeutic strategy for diabetes‐related ED and a target for the treatment of diabetes‐related conditions with functionalized nanoparticles to regulate mitophagy.
中文翻译:

一种靶向线粒体的压电纳米系统,通过自噬调节治疗勃起功能障碍
由外部刺激(如高血糖水平和炎症)引起的线粒体损伤会导致活性氧 (ROS) 产生过多。现有的抗氧化剂只能清除 ROS,不能解决 ROS 产生的根本原因,即异常的线粒体。为了克服这一限制,该研究开发了一种针对线粒体的压电协同载药纳米系统 (BaTCG nanosystem)。BaTCG 纳米系统通过三苯基膦修饰输送到线粒体,并在超声刺激下产生电流,从而促进线粒体自噬并恢复线粒体稳态。在糖尿病相关勃起功能障碍 (ED) 模型中,BaTCG 纳米系统通过压电效应诱导的电流,不仅促进线粒体自噬,从而减少 ROS 的产生,还释放长效胰高血糖素样肽-1 受体激动剂 (GLP-1RAs),有效降低血糖水平和线粒体损伤。该纳米系统的每个组成部分都单独或协同发挥作用,从而促进海绵体修复和恢复勃起功能。总之,这些发现为糖尿病相关 ED 提供了一种新的治疗策略,并为用功能化纳米颗粒调节线粒体自噬治疗糖尿病相关疾病提供了靶点。
更新日期:2024-12-17
中文翻译:

一种靶向线粒体的压电纳米系统,通过自噬调节治疗勃起功能障碍
由外部刺激(如高血糖水平和炎症)引起的线粒体损伤会导致活性氧 (ROS) 产生过多。现有的抗氧化剂只能清除 ROS,不能解决 ROS 产生的根本原因,即异常的线粒体。为了克服这一限制,该研究开发了一种针对线粒体的压电协同载药纳米系统 (BaTCG nanosystem)。BaTCG 纳米系统通过三苯基膦修饰输送到线粒体,并在超声刺激下产生电流,从而促进线粒体自噬并恢复线粒体稳态。在糖尿病相关勃起功能障碍 (ED) 模型中,BaTCG 纳米系统通过压电效应诱导的电流,不仅促进线粒体自噬,从而减少 ROS 的产生,还释放长效胰高血糖素样肽-1 受体激动剂 (GLP-1RAs),有效降低血糖水平和线粒体损伤。该纳米系统的每个组成部分都单独或协同发挥作用,从而促进海绵体修复和恢复勃起功能。总之,这些发现为糖尿病相关 ED 提供了一种新的治疗策略,并为用功能化纳米颗粒调节线粒体自噬治疗糖尿病相关疾病提供了靶点。






























 京公网安备 11010802027423号
京公网安备 11010802027423号