当前位置:
X-MOL 学术
›
Adv. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Matrix Viscoelasticity Controls Differentiation of Human Blood Vessel Organoids into Arterioles and Promotes Neovascularization in Myocardial Infarction
Advanced Materials ( IF 27.4 ) Pub Date : 2024-12-17 , DOI: 10.1002/adma.202410802 Dayu Sun, Kunyu Zhang, Feiyang Zheng, Guanyuan Yang, Mingcan Yang, Youqian Xu, Yinhua Qin, Mingxin Lin, Yanzhao Li, Ju Tan, Qiyu Li, Xiaohang Qu, Gang Li, Liming Bian, Chuhong Zhu
Advanced Materials ( IF 27.4 ) Pub Date : 2024-12-17 , DOI: 10.1002/adma.202410802 Dayu Sun, Kunyu Zhang, Feiyang Zheng, Guanyuan Yang, Mingcan Yang, Youqian Xu, Yinhua Qin, Mingxin Lin, Yanzhao Li, Ju Tan, Qiyu Li, Xiaohang Qu, Gang Li, Liming Bian, Chuhong Zhu
|
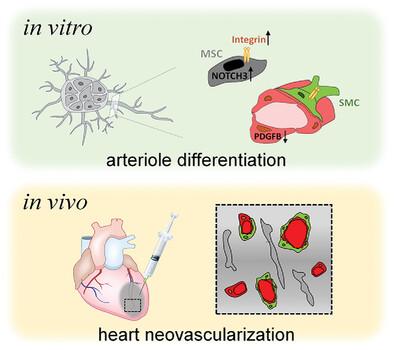
Stem cell‐derived blood vessel organoids are embedded in extracellular matrices to stimulate vessel sprouting. Although vascular organoids in 3D collagen I‐Matrigel gels are currently available, they are primarily capillaries composed of endothelial cells (ECs), pericytes, and mesenchymal stem‐like cells, which necessitate mature arteriole differentiation for neovascularization. In this context, the hypothesis that matrix viscoelasticity regulates vascular development is investigated in 3D cultures by encapsulating blood vessel organoids within viscoelastic gelatin/β‐CD assembly dynamic hydrogels or methacryloyl gelatin non‐dynamic hydrogels. The vascular organoids within the dynamic hydrogel demonstrate enhanced angiogenesis and differentiation into arterioles containing smooth muscle cells. The dynamic hydrogel mechanical microenvironment promotes vascular patterning and arteriolar differentiation by elevating notch receptor 3 signaling in mesenchymal stem cells and downregulating platelet‐derived growth factor B expression in ECs. Transplantation of vascular organoids in vivo, along with the dynamic hydrogel, leads to the reassembly of arterioles and restoration of cardiac function in infarcted hearts. These findings indicate that the viscoelastic properties of the matrix play a crucial role in controlling the vascular organization and differentiation processes, suggesting an exciting potential for its application in regenerative medicine.
中文翻译:

基质粘弹性控制人血管类器官向小动脉的分化并促进心肌梗死的新生血管形成
干细胞来源的血管类器官嵌入细胞外基质中以刺激血管发芽。尽管目前有 3D 胶原蛋白 I-Matrigel 凝胶中的血管类器官可用,但它们主要是由内皮细胞 (EC)、周细胞和间充质干细胞样细胞组成的毛细血管,这需要成熟的小动脉分化才能进行新生血管形成。在这种情况下,通过将血管类器官封装在粘弹性明胶/β-CD 组装动态水凝胶或甲基丙烯酰明胶非动态水凝胶中,在 3D 培养中研究了基质粘弹性调节血管发育的假设。动态水凝胶内的血管类器官表现出增强的血管生成和分化为含有平滑肌细胞的小动脉。动态水凝胶机械微环境通过提高间充质干细胞中的 Notch 受体 3 信号传导和下调 ECs 中血小板衍生生长因子 B 的表达来促进血管模式化和小动脉分化。体内血管类器官的移植以及动态水凝胶导致梗死心脏中小动脉的重组和心脏功能的恢复。这些发现表明,基质的粘弹性在控制血管组织和分化过程中起着至关重要的作用,表明其在再生医学中的应用具有令人兴奋的潜力。
更新日期:2024-12-17
中文翻译:

基质粘弹性控制人血管类器官向小动脉的分化并促进心肌梗死的新生血管形成
干细胞来源的血管类器官嵌入细胞外基质中以刺激血管发芽。尽管目前有 3D 胶原蛋白 I-Matrigel 凝胶中的血管类器官可用,但它们主要是由内皮细胞 (EC)、周细胞和间充质干细胞样细胞组成的毛细血管,这需要成熟的小动脉分化才能进行新生血管形成。在这种情况下,通过将血管类器官封装在粘弹性明胶/β-CD 组装动态水凝胶或甲基丙烯酰明胶非动态水凝胶中,在 3D 培养中研究了基质粘弹性调节血管发育的假设。动态水凝胶内的血管类器官表现出增强的血管生成和分化为含有平滑肌细胞的小动脉。动态水凝胶机械微环境通过提高间充质干细胞中的 Notch 受体 3 信号传导和下调 ECs 中血小板衍生生长因子 B 的表达来促进血管模式化和小动脉分化。体内血管类器官的移植以及动态水凝胶导致梗死心脏中小动脉的重组和心脏功能的恢复。这些发现表明,基质的粘弹性在控制血管组织和分化过程中起着至关重要的作用,表明其在再生医学中的应用具有令人兴奋的潜力。






























 京公网安备 11010802027423号
京公网安备 11010802027423号