当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Metal-Free Synthesis of 4-Bromoisoquinolines through Brominative Annulation of 2-Alkynyl Arylimidate Using In Situ-Generated Transient Bromoiodane
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-12-16 , DOI: 10.1021/acs.joc.4c02867 Akshay S. Pathare, Sermadurai Selvakumar
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-12-16 , DOI: 10.1021/acs.joc.4c02867 Akshay S. Pathare, Sermadurai Selvakumar
|
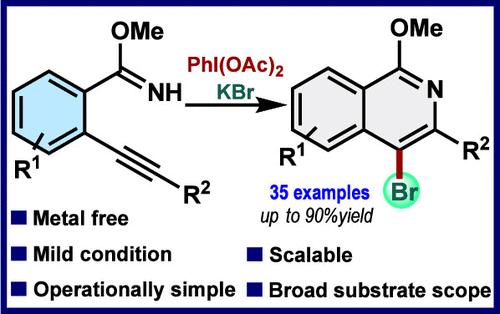
Herein, we report the in situ-generated transient bromoiodane-mediated brominative annulation of 2-alkynyl arylimidate for the synthesis of 4-bromoisoquinolines at room temperature. Using a simple hypervalent iodine reagent PIDA as a mild oxidant and potassium bromide as the halogen source, a broad range of valuable 4-bromoisoquinolines can be synthesized in excellent yields. The reaction features readily available chemicals, mild metal-free conditions, and high functional group tolerance, providing an efficient alternative for the construction of halogenated isoquinolines.
中文翻译:

使用原位生成的瞬时溴碘化通过 2-炔基芳基酯的溴化环化无金属合成 4-溴异喹啉
在此,我们报道了原位产生的瞬时溴碘介导的 2-炔基芳基丙烯酸酯的溴化环化,用于在室温下合成 4-溴异喹啉。使用简单的高价碘试剂 PIDA 作为温和氧化剂,溴化钾作为卤素源,可以以极高的产率合成多种有价值的 4-溴异喹啉。该反应具有易得化学品、温和的无金属条件和高官能团耐受性,为构建卤代异喹啉提供了一种有效的替代方案。
更新日期:2024-12-17
中文翻译:

使用原位生成的瞬时溴碘化通过 2-炔基芳基酯的溴化环化无金属合成 4-溴异喹啉
在此,我们报道了原位产生的瞬时溴碘介导的 2-炔基芳基丙烯酸酯的溴化环化,用于在室温下合成 4-溴异喹啉。使用简单的高价碘试剂 PIDA 作为温和氧化剂,溴化钾作为卤素源,可以以极高的产率合成多种有价值的 4-溴异喹啉。该反应具有易得化学品、温和的无金属条件和高官能团耐受性,为构建卤代异喹啉提供了一种有效的替代方案。






























 京公网安备 11010802027423号
京公网安备 11010802027423号