当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Scalable Process Development of Ceritinib: Application of Statistical Design of Experiments
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-12-17 , DOI: 10.1021/acs.oprd.4c00416 Shravan Kumar Komati, Amarendhar Manda, Sridhar Vasam, Gopal Chandru Senadi, Arthanareeswari Maruthapillai, Rakeshwar Bandichhor
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-12-17 , DOI: 10.1021/acs.oprd.4c00416 Shravan Kumar Komati, Amarendhar Manda, Sridhar Vasam, Gopal Chandru Senadi, Arthanareeswari Maruthapillai, Rakeshwar Bandichhor
|
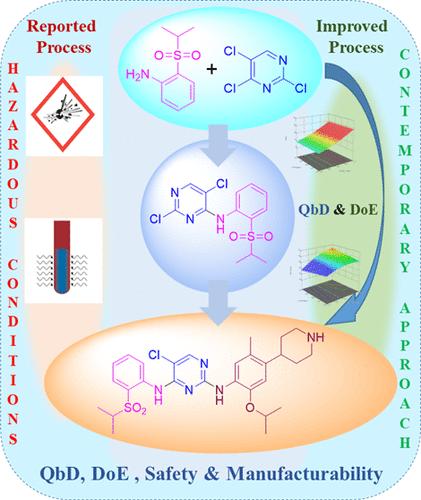
A convenient and commercially viable cost-effective, safe manufacturing process was developed to manufacture ceritinib. This work describes the implementation of the quality by design approach through the identification of critical quality attributes (CQAs), critical material attributes (CMAs), and critical process parameters (CPPs). Application of the statistical design of experimentation for an experimental plan to find the relationship between CQAs, CMAs, and CPPs. This work also describes a superior process for large-scale manufacturing of ceritinib in terms of process safety, handling, scalability, and enhanced throughput. Work captured here addressed the challenges in reported procedures such as sodium hydride’s explosive hazard, high-temperature microwave conditions, nitro to amine reduction under high pressure using a palladium catalyst, and column chromatography to purify the finished product.
中文翻译:

Ceritinib 的可扩展工艺开发:实验统计设计的应用
开发了一种方便且商业上可行的经济高效、安全的制造工艺来生产 ceritinib。这项工作描述了通过识别关键质量属性 (CQA)、关键材料属性 (CMA) 和关键过程参数 (CPP) 来实施质量源于设计方法。将实验的统计设计应用于实验计划,以发现 CQA、CMA 和 CPP 之间的关系。这项工作还描述了一种在工艺安全性、处理、可扩展性和增强通量方面大规模生产 ceritinib 的卓越工艺。此处捕获的工作解决了所报道程序中的挑战,例如氢化钠的爆炸危险、高温微波条件、使用钯催化剂在高压下将硝基还原为胺以及柱色谱纯化成品。
更新日期:2024-12-17
中文翻译:

Ceritinib 的可扩展工艺开发:实验统计设计的应用
开发了一种方便且商业上可行的经济高效、安全的制造工艺来生产 ceritinib。这项工作描述了通过识别关键质量属性 (CQA)、关键材料属性 (CMA) 和关键过程参数 (CPP) 来实施质量源于设计方法。将实验的统计设计应用于实验计划,以发现 CQA、CMA 和 CPP 之间的关系。这项工作还描述了一种在工艺安全性、处理、可扩展性和增强通量方面大规模生产 ceritinib 的卓越工艺。此处捕获的工作解决了所报道程序中的挑战,例如氢化钠的爆炸危险、高温微波条件、使用钯催化剂在高压下将硝基还原为胺以及柱色谱纯化成品。






























 京公网安备 11010802027423号
京公网安备 11010802027423号