当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of an Isomerized Bithiazole Imide (iBTzI) Acceptor and π-Extension via Intramolecular Noncovalent Interactions
Organic Letters ( IF 4.9 ) Pub Date : 2024-12-16 , DOI: 10.1021/acs.orglett.4c04205 Lanen Wei, Kunhan Xu, Ting Qi
Organic Letters ( IF 4.9 ) Pub Date : 2024-12-16 , DOI: 10.1021/acs.orglett.4c04205 Lanen Wei, Kunhan Xu, Ting Qi
|
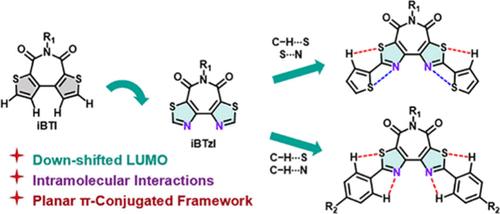
An isomerized bithiazole imide (iBTzI) acceptor was effectively synthesized and functionalized via Suzuki or Stille coupling reactions. Compared with the isomerized bithiophene imide (iBTI) and bithiazole imide (BTzI), iBTzI has a more planar skeleton. Furthermore, the conjugated skeleton of iBTzI can be extended by hydrogen and chalcogen bonds. The donor–acceptor–donor-type (D–A–D-type) iBTzI derivatives display blue-to-red emission and enhanced photoluminescence quantum yields (ΦPLs) in solution, showing great promise in luminous materials.
中文翻译:

通过分子内非共价相互作用合成异构化联噻唑酰亚胺 (iBTzI) 受体和π延伸
异构化的二噻唑酰亚胺 (iBTzI) 受体通过 Suzuki 或 Stille 偶联反应有效合成和功能化。与异构化联噻吩酰亚胺 (iBTI) 和双噻唑酰亚胺 (BTzI) 相比,iBTzI 具有更平面的骨架。此外,iBTzI 的共轭骨架可以通过氢键和硫属键延伸。供体-受体-供体型 (D-A-D 型) iBTzI 衍生物在溶液中表现出蓝到红的发射和增强的光致发光量子产率 (ΦPLs),在发光材料中显示出巨大的前景。
更新日期:2024-12-17
中文翻译:

通过分子内非共价相互作用合成异构化联噻唑酰亚胺 (iBTzI) 受体和π延伸
异构化的二噻唑酰亚胺 (iBTzI) 受体通过 Suzuki 或 Stille 偶联反应有效合成和功能化。与异构化联噻吩酰亚胺 (iBTI) 和双噻唑酰亚胺 (BTzI) 相比,iBTzI 具有更平面的骨架。此外,iBTzI 的共轭骨架可以通过氢键和硫属键延伸。供体-受体-供体型 (D-A-D 型) iBTzI 衍生物在溶液中表现出蓝到红的发射和增强的光致发光量子产率 (ΦPLs),在发光材料中显示出巨大的前景。






























 京公网安备 11010802027423号
京公网安备 11010802027423号