当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Staudinger Cleavages of Amides on Naphthalene for the Ipsilateral Effect of 1,8-Substituents
Organic Letters ( IF 4.9 ) Pub Date : 2024-12-16 , DOI: 10.1021/acs.orglett.4c04337 Lunyu Ou, Zhengyi Yi, Yue Zhang, Yufen Zhao, Hua Fu
Organic Letters ( IF 4.9 ) Pub Date : 2024-12-16 , DOI: 10.1021/acs.orglett.4c04337 Lunyu Ou, Zhengyi Yi, Yue Zhang, Yufen Zhao, Hua Fu
|
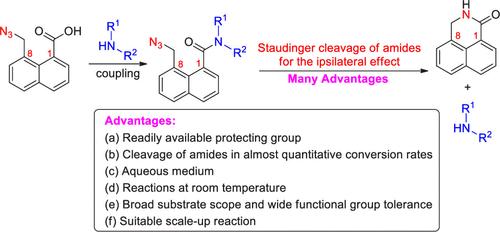
8-(Azidomethyl)-1-naphthoic acid was elaborately prepared, and its coupling with amines provided the corresponding 8-(azidomethyl)-1-naphthamides. The Staudinger reactions of 8-(azidomethyl)-1-naphthamides with phosphine produced iminophosphoranes, and easy intramolecular cyclization of the iminophosphoranes afforded 2,3-dihydro-1H-benzo[de]isoquinolin-1-one leaving amines with almost quantitative conversion rates for the ipsilateral effect of 1,8-substituents on naphthalene. The protocol exhibits some advantages, including a readily available protecting group, cleavages of amides in almost quantitative conversion rates, an aqueous medium, reactions at room temperature, a broad substrate scope, wide functional group tolerance, and suitable scale-up reactions.
中文翻译:

酰胺对萘的 Staudinger 裂解对 1,8-取代基的同侧效应
精心制备了 8-(叠氮甲基)-1-萘甲酸,它与胺的偶联提供了相应的 8-(叠氮甲基)-1-萘酰胺。8-(叠氮甲基)-1-萘酰胺与膦的 Staudinger 反应产生了亚氨基腈烷,亚氨基腈烷的易分子内环化得到了 2,3-二氢-1H-苯并[去]异喹啉-1-酮,使胺具有几乎定量的转化率,用于 1,8-取代基对萘的同侧作用。该方案具有一些优点,包括现成的保护基团、以近乎定量的转化率切割酰胺、水性介质、室温反应、广泛的底物范围、广泛的官能团耐受性和合适的放大反应。
更新日期:2024-12-17
中文翻译:

酰胺对萘的 Staudinger 裂解对 1,8-取代基的同侧效应
精心制备了 8-(叠氮甲基)-1-萘甲酸,它与胺的偶联提供了相应的 8-(叠氮甲基)-1-萘酰胺。8-(叠氮甲基)-1-萘酰胺与膦的 Staudinger 反应产生了亚氨基腈烷,亚氨基腈烷的易分子内环化得到了 2,3-二氢-1H-苯并[去]异喹啉-1-酮,使胺具有几乎定量的转化率,用于 1,8-取代基对萘的同侧作用。该方案具有一些优点,包括现成的保护基团、以近乎定量的转化率切割酰胺、水性介质、室温反应、广泛的底物范围、广泛的官能团耐受性和合适的放大反应。






























 京公网安备 11010802027423号
京公网安备 11010802027423号