Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Self-Assembled Bolaamphiphile-Based Organic Nanotubes as Efficient Cu(II) Ion Adsorbents
Langmuir ( IF 3.7 ) Pub Date : 2024-12-16 , DOI: 10.1021/acs.langmuir.4c03619 Takashi Ito, S. Erin Frenk, Nikhil Rai, Soenke Seifert, Xiao-Min Lin, Srikanth Nayak
Langmuir ( IF 3.7 ) Pub Date : 2024-12-16 , DOI: 10.1021/acs.langmuir.4c03619 Takashi Ito, S. Erin Frenk, Nikhil Rai, Soenke Seifert, Xiao-Min Lin, Srikanth Nayak
|
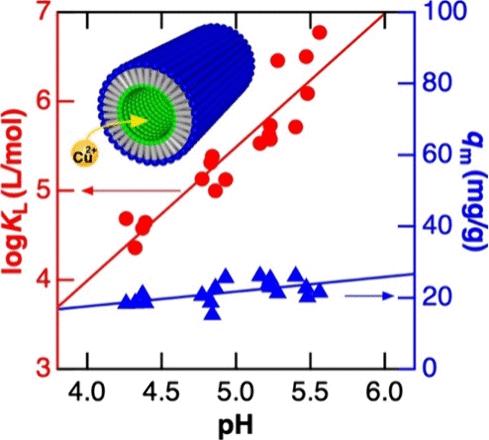
Self-assembled organic nanotubes (ONTs) have been actively examined for various applications such as chemical separations and catalysis owing to their well-defined tubular nanostructures with distinct chemical environments at the wall and internal/external surfaces. Adsorption of heavy metal ions onto ONTs plays an essential role in many of these applications but has rarely been assessed quantitatively. Herein, we investigated interactions between Cu2+ and single-/quadruple-wall bolaamphiphile-based ONTs having inner carboxyl groups with different inner diameters, COOH-ONT10nm and COOH-ONT20nm. We first examined the effects of Cu2+ on their nanotubular structures using SAXS, STEM, and AFM. COOH-ONT10nm was stable in aqueous Cu2+ solution in contrast to COOH-ONT20nm owing to the presence of polyglycine-II-type hydrogen bonding networks within its wall. Subsequently, we studied the Cu2+ adsorption behavior of COOH-ONT10nm by monitoring the concentration of unbound Cu2+ using linear sweep anodic stripping voltammetry. The Cu2+ adsorption was quick, attributable to efficient Cu2+ partitioning through the open ends of the ONT, followed by fast Cu2+ diffusion in the uniform, relatively large nanochannel. More importantly, the Cu2+ adsorption capacity and affinity of COOH-ONT10nm were measured under different pH conditions using the Langmuir adsorption model. The adsorption capacity was similar at the pH range examined, showing the participation of approximately 25% of the inner carboxyl groups in the adsorption. The adsorption affinity increased with pH, indicating the essential role of the deprotonated carboxyl groups in Cu2+ adsorption. Most interestingly, the Langmuir adsorption constant was significantly higher than those of previously reported synthetic adsorbents and planar monolayer based on carboxyl binding sites. The high Cu2+ affinity of the ONT was attributable to the highly dense binding sites on the well-defined nanoscale concave structure of the inner channel. These results provide a valuable guideline for designing self-assembled nanomaterials for efficient chemical separations, detection, and catalysis.
中文翻译:

自组装的基于玻色两亲物的有机纳米管作为高效的 Cu(II) 离子吸附剂
自组装有机纳米管 (ONT) 因其定义明确的管状纳米结构在壁和内/外表面具有不同的化学环境而被广泛研究用于化学分离和催化等各种应用。重金属离子对 ONT 的吸附在许多此类应用中起着至关重要的作用,但很少进行定量评估。在此,我们研究了 Cu2+ 和基于单壁/四壁两亲物的 ONT 之间的相互作用,这些 ONT 具有不同内径的内羧基,COOH-ONT10nm 和 COOH-ONT20nm。我们首先使用 SAXS、STEM 和 AFM 研究了 Cu2+ 对其纳米管结构的影响。与 COOH-ONT20nm 相比,COOH-ONT10nm 在 Cu2+ 水溶液中是稳定的,因为其壁内存在聚甘氨酸-II 型氢键网络。随后,我们通过使用线性扫描阳极溶出伏安法监测未结合的 Cu2+ 的浓度,研究了 COOH-ONT10nm 的 Cu2+ 吸附行为。Cu2+ 吸附很快,这归因于 Cu2+ 通过 ONT 开口端的高效分配,然后 Cu2+ 在均匀的、相对较大的纳米通道中快速扩散。更重要的是,使用 Langmuir 吸附模型在不同 pH 条件下测量了 Cu2+ 吸附容量和 COOH-ONT10nm 的亲和力。在检查的 pH 范围内,吸附能力相似,表明大约 25% 的内羧基参与吸附。 吸附亲和力随 pH 值的增加而增加,表明去质子化的羧基在 Cu2+ 吸附中起着重要作用。最有趣的是,Langmuir 吸附常数显著高于先前报道的合成吸附剂和基于羧基结合位点的平面单层吸附常数。ONT 的高 Cu2+ 亲和力归因于内通道明确定义的纳米级凹结构上的高密度结合位点。这些结果为设计用于高效化学分离、检测和催化的自组装纳米材料提供了有价值的指导。
更新日期:2024-12-17
中文翻译:

自组装的基于玻色两亲物的有机纳米管作为高效的 Cu(II) 离子吸附剂
自组装有机纳米管 (ONT) 因其定义明确的管状纳米结构在壁和内/外表面具有不同的化学环境而被广泛研究用于化学分离和催化等各种应用。重金属离子对 ONT 的吸附在许多此类应用中起着至关重要的作用,但很少进行定量评估。在此,我们研究了 Cu2+ 和基于单壁/四壁两亲物的 ONT 之间的相互作用,这些 ONT 具有不同内径的内羧基,COOH-ONT10nm 和 COOH-ONT20nm。我们首先使用 SAXS、STEM 和 AFM 研究了 Cu2+ 对其纳米管结构的影响。与 COOH-ONT20nm 相比,COOH-ONT10nm 在 Cu2+ 水溶液中是稳定的,因为其壁内存在聚甘氨酸-II 型氢键网络。随后,我们通过使用线性扫描阳极溶出伏安法监测未结合的 Cu2+ 的浓度,研究了 COOH-ONT10nm 的 Cu2+ 吸附行为。Cu2+ 吸附很快,这归因于 Cu2+ 通过 ONT 开口端的高效分配,然后 Cu2+ 在均匀的、相对较大的纳米通道中快速扩散。更重要的是,使用 Langmuir 吸附模型在不同 pH 条件下测量了 Cu2+ 吸附容量和 COOH-ONT10nm 的亲和力。在检查的 pH 范围内,吸附能力相似,表明大约 25% 的内羧基参与吸附。 吸附亲和力随 pH 值的增加而增加,表明去质子化的羧基在 Cu2+ 吸附中起着重要作用。最有趣的是,Langmuir 吸附常数显著高于先前报道的合成吸附剂和基于羧基结合位点的平面单层吸附常数。ONT 的高 Cu2+ 亲和力归因于内通道明确定义的纳米级凹结构上的高密度结合位点。这些结果为设计用于高效化学分离、检测和催化的自组装纳米材料提供了有价值的指导。






























 京公网安备 11010802027423号
京公网安备 11010802027423号