当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Noncovalent Construction of Hangman Cobalt Phthalocyanine for Enhanced Electrochemical Carbon Dioxide Reduction
Chemistry of Materials ( IF 7.2 ) Pub Date : 2024-12-16 , DOI: 10.1021/acs.chemmater.4c02697 Ye Zhou, Xiaoyue Duan, Xin Xu, Poe Ei Phyu Win, Shi-Bin Ren, Jiong Wang
Chemistry of Materials ( IF 7.2 ) Pub Date : 2024-12-16 , DOI: 10.1021/acs.chemmater.4c02697 Ye Zhou, Xiaoyue Duan, Xin Xu, Poe Ei Phyu Win, Shi-Bin Ren, Jiong Wang
|
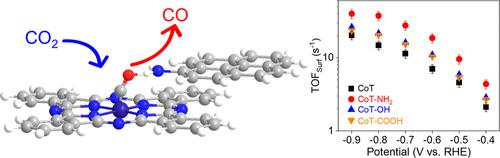
The hangman structure plays a critical role in determining the reaction rates of molecular CO2 electrocatalysis through constructing pendant functional groups in secondary coordination spheres of metal active sites. However, achieving hangman structures commonly requires complicated asymmetric synthesis. It is necessary to search for simple alternative strategies to develop hangman molecular electrocatalysis with realization of the concept of green chemistry. In this work, we report the synthesis of hangman molecular electrocatalysts based on the noncovalent π–π interaction between cobalt (Co) phthalocyanine nanotubes and 1-aminopyrene. It promoted the kinetics of interfacial inner and outer sphere electron transfer on the complex and chemisorption of *COOH and *CO species through interaction with both Co sites and pendant amine groups in a bridge geometry. The resultant Co sites afforded a very high turnover frequency of 4.37 s–1 at an overpotential of 0.29 V for electrochemical CO2 to CO conversion and thus afforded an industrial interest current density being steady at 350 mA cm–2.
中文翻译:

Hangman 钴酞菁的非共价构建用于增强电化学二氧化碳还原
刽子结构通过在金属活性位点的次级配位球中构建悬垂官能团,在决定分子 CO2 电催化的反应速率中起着关键作用。然而,实现 hangman 结构通常需要复杂的不对称合成。有必要寻找简单的替代策略来开发 hangman 分子电催化并实现绿色化学的概念。在这项工作中,我们报道了基于钴 (Co) 酞菁纳米管和 1-氨基芘之间的非共价 π-π 相互作用的 hangman 分子电催化剂的合成。它通过与电桥几何形状中的 Co 位点和悬垂胺基团相互作用,促进了界面内球和外球电子转移对络合物的动力学以及 *COOH 和 *CO 物种的化学吸附。所得 Co 位点在 0.29 V 的过电位下提供了 4.37 s–1 的非常高的周转频率,用于电化学 CO2 到 CO 的转换,因此提供了稳定在 350 mA cm–2 的工业关注电流密度。
更新日期:2024-12-17
中文翻译:

Hangman 钴酞菁的非共价构建用于增强电化学二氧化碳还原
刽子结构通过在金属活性位点的次级配位球中构建悬垂官能团,在决定分子 CO2 电催化的反应速率中起着关键作用。然而,实现 hangman 结构通常需要复杂的不对称合成。有必要寻找简单的替代策略来开发 hangman 分子电催化并实现绿色化学的概念。在这项工作中,我们报道了基于钴 (Co) 酞菁纳米管和 1-氨基芘之间的非共价 π-π 相互作用的 hangman 分子电催化剂的合成。它通过与电桥几何形状中的 Co 位点和悬垂胺基团相互作用,促进了界面内球和外球电子转移对络合物的动力学以及 *COOH 和 *CO 物种的化学吸附。所得 Co 位点在 0.29 V 的过电位下提供了 4.37 s–1 的非常高的周转频率,用于电化学 CO2 到 CO 的转换,因此提供了稳定在 350 mA cm–2 的工业关注电流密度。






























 京公网安备 11010802027423号
京公网安备 11010802027423号