当前位置:
X-MOL 学术
›
Chem. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Octadecanoids: Synthesis and Bioactivity of 18-Carbon Oxygenated Fatty Acids in Mammals, Bacteria, and Fungi
Chemical Reviews ( IF 51.4 ) Pub Date : 2024-12-16 , DOI: 10.1021/acs.chemrev.3c00520 Johanna Revol-Cavalier, Alessandro Quaranta, John W. Newman, Alan R. Brash, Mats Hamberg, Craig E. Wheelock
Chemical Reviews ( IF 51.4 ) Pub Date : 2024-12-16 , DOI: 10.1021/acs.chemrev.3c00520 Johanna Revol-Cavalier, Alessandro Quaranta, John W. Newman, Alan R. Brash, Mats Hamberg, Craig E. Wheelock
|
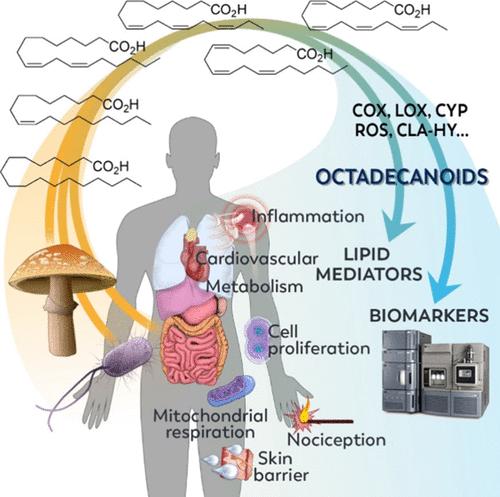
The octadecanoids are a broad class of lipids consisting of the oxygenated products of 18-carbon fatty acids. Originally referring to production of the phytohormone jasmonic acid, the octadecanoid pathway has been expanded to include products of all 18-carbon fatty acids. Octadecanoids are formed biosynthetically in mammals via cyclooxygenase (COX), lipoxygenase (LOX), and cytochrome P450 (CYP) activity, as well as nonenzymatically by photo- and autoxidation mechanisms. While octadecanoids are well-known mediators in plants, their role in the regulation of mammalian biological processes has been generally neglected. However, there have been significant advancements in recognizing the importance of these compounds in mammals and their involvement in the mediation of inflammation, nociception, and cell proliferation, as well as in immuno- and tissue modulation, coagulation processes, hormone regulation, and skin barrier formation. More recently, the gut microbiome has been shown to be a significant source of octadecanoid biosynthesis, providing additional biosynthetic routes including hydratase activity (e.g., CLA-HY, FA-HY1, FA-HY2). In this review, we summarize the current field of octadecanoids, propose standardized nomenclature, provide details of octadecanoid preparation and measurement, summarize the phase-I metabolic pathway of octadecanoid formation in mammals, bacteria, and fungi, and describe their biological activity in relation to mammalian pathophysiology as well as their potential use as biomarkers of health and disease.
中文翻译:

十八烷类化合物:哺乳动物、细菌和真菌中 18 碳含氧脂肪酸的合成和生物活性
十八烷类化合物是一大类脂质,由 18 碳脂肪酸的含氧产物组成。十八烷类化合物途径最初是指植物激素茉莉酸的产生,现已扩展到包括所有 18 碳脂肪酸的产物。十八烷类化合物在哺乳动物中通过环氧合酶 (COX)、脂氧合酶 (LOX) 和细胞色素 P450 (CYP) 活性生物合成形成,以及通过光氧化和自氧化机制以非酶促方式形成。虽然十八烷类化合物是植物中众所周知的介质,但它们在哺乳动物生物过程调节中的作用通常被忽视。然而,在认识到这些化合物在哺乳动物中的重要性及其参与炎症、伤害感受和细胞增殖以及免疫和组织调节、凝血过程、激素调节和皮肤屏障形成方面已经取得了重大进展。最近,肠道微生物组已被证明是十八烷类化合物生物合成的重要来源,提供额外的生物合成途径,包括水合酶活性(例如 CLA-HY、FA-HY1、FA-HY2)。在这篇综述中,我们总结了当前十八进制药物领域,提出了标准化命名法,提供了十八进制和测量的详细信息,总结了哺乳动物、细菌和真菌中十八进制物质形成的 I 期代谢途径,并描述了它们与哺乳动物病理生理学相关的生物活性以及它们作为健康和疾病生物标志物的潜在用途。
更新日期:2024-12-17
中文翻译:

十八烷类化合物:哺乳动物、细菌和真菌中 18 碳含氧脂肪酸的合成和生物活性
十八烷类化合物是一大类脂质,由 18 碳脂肪酸的含氧产物组成。十八烷类化合物途径最初是指植物激素茉莉酸的产生,现已扩展到包括所有 18 碳脂肪酸的产物。十八烷类化合物在哺乳动物中通过环氧合酶 (COX)、脂氧合酶 (LOX) 和细胞色素 P450 (CYP) 活性生物合成形成,以及通过光氧化和自氧化机制以非酶促方式形成。虽然十八烷类化合物是植物中众所周知的介质,但它们在哺乳动物生物过程调节中的作用通常被忽视。然而,在认识到这些化合物在哺乳动物中的重要性及其参与炎症、伤害感受和细胞增殖以及免疫和组织调节、凝血过程、激素调节和皮肤屏障形成方面已经取得了重大进展。最近,肠道微生物组已被证明是十八烷类化合物生物合成的重要来源,提供额外的生物合成途径,包括水合酶活性(例如 CLA-HY、FA-HY1、FA-HY2)。在这篇综述中,我们总结了当前十八进制药物领域,提出了标准化命名法,提供了十八进制和测量的详细信息,总结了哺乳动物、细菌和真菌中十八进制物质形成的 I 期代谢途径,并描述了它们与哺乳动物病理生理学相关的生物活性以及它们作为健康和疾病生物标志物的潜在用途。






























 京公网安备 11010802027423号
京公网安备 11010802027423号