当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electronic Regulation of Pt for Low-Temperature Hydrogen Generation from Methanol and Water
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2024-12-16 , DOI: 10.1021/acssuschemeng.4c07671 Qiankang Liao, You Wang, Chen Chen, Sai Zhang
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2024-12-16 , DOI: 10.1021/acssuschemeng.4c07671 Qiankang Liao, You Wang, Chen Chen, Sai Zhang
|
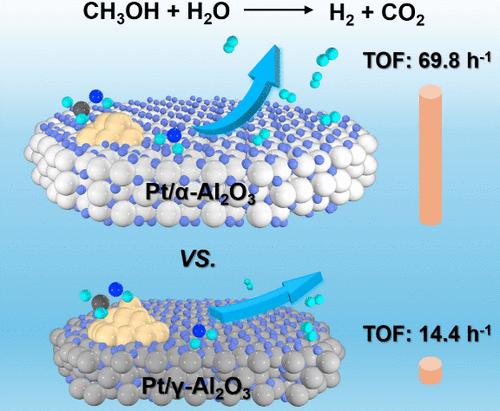
The aqueous-phase reforming of the methanol (APRM) reaction provides a potential approach for hydrogen (H2) storage and transportation. However, the limited capacity of Pt nanocatalysts for H2O activation leads to the drawback of requiring high reaction temperatures (>200 °C) to achieve efficient H2 generation through the APRM reaction. Herein, the electronic density of Pt nanocatalysts has been regulated by the phase of the Al2O3 supports. Mechanism analysis revealed that the α-Al2O3 supports with larger lattice fringe spacing resulted in an enhanced electronic density of Pt nanocatalysts, thereby enabling the effective adsorption and activation of H2O. Consequently, the Pt/α-Al2O3 catalysts exhibited a TOF value of 69.8 h–1 at 30 °C for H2 generation via APRM reaction. Notably, this H2 generation rate even suppressed that achieved by previous state-of-the-art homogeneous catalysts. This finding presents a promising avenue toward flexible hydrogen utilization.
中文翻译:

甲醇和水低温制氢的 Pt 的电子调节
甲醇 (APRM) 反应的水相重整为氢气 (H2) 的储存和运输提供了一种潜在的方法。然而,Pt 纳米催化剂对 H2O 活化的能力有限,导致需要高反应温度 (>200 °C) 才能通过 APRM 反应实现高效 H2 生成的缺点。在此,Pt 纳米催化剂的电子密度受 Al2O3 载体相的调节。机理分析表明,具有较大晶格条纹间距的 α-Al2O3 载体导致 Pt 纳米催化剂的电子密度增加,从而能够有效吸附和活化 H2O。因此,Pt/α-Al2O3 催化剂在 30 °C 下通过 APRM 反应生成 H2 的 TOF 值为 69.8 h–1。值得注意的是,这种 H2 生成速率甚至抑制了以前最先进的均相催化剂所实现的速率。这一发现为灵活的氢利用提供了一条有前途的途径。
更新日期:2024-12-16
中文翻译:

甲醇和水低温制氢的 Pt 的电子调节
甲醇 (APRM) 反应的水相重整为氢气 (H2) 的储存和运输提供了一种潜在的方法。然而,Pt 纳米催化剂对 H2O 活化的能力有限,导致需要高反应温度 (>200 °C) 才能通过 APRM 反应实现高效 H2 生成的缺点。在此,Pt 纳米催化剂的电子密度受 Al2O3 载体相的调节。机理分析表明,具有较大晶格条纹间距的 α-Al2O3 载体导致 Pt 纳米催化剂的电子密度增加,从而能够有效吸附和活化 H2O。因此,Pt/α-Al2O3 催化剂在 30 °C 下通过 APRM 反应生成 H2 的 TOF 值为 69.8 h–1。值得注意的是,这种 H2 生成速率甚至抑制了以前最先进的均相催化剂所实现的速率。这一发现为灵活的氢利用提供了一条有前途的途径。































 京公网安备 11010802027423号
京公网安备 11010802027423号