当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Efficient Cellobiose Hydrolysis over a Sulfonated Carbon Catalyst in a Spatially Separated Microwave Electric- and Magnetic-Field Flow Reactor
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2024-12-16 , DOI: 10.1021/acssuschemeng.4c07690 Shuntaro Tsubaki, Kazuaki Senda, Ayumu Onda, Satoshi Fujii
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2024-12-16 , DOI: 10.1021/acssuschemeng.4c07690 Shuntaro Tsubaki, Kazuaki Senda, Ayumu Onda, Satoshi Fujii
|
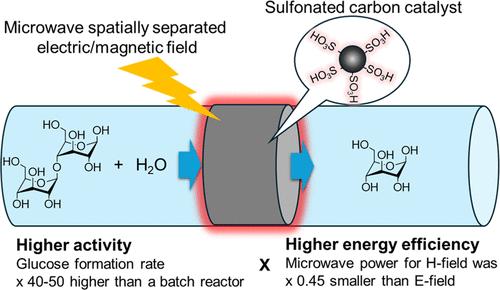
Enhanced polysaccharide hydrolysis is essential for converting polysaccharides into mono- and oligosaccharide sugars for use in food, pharmaceutical, and biobased chemical applications. In this study, we developed an efficient continuous-flow hydrolysis process by applying microwaves and sulfonated carbon catalyst (AC-SO3H) using cellobiose as a model sugar substrate. We built a microwave flow reactor equipped with a rectangular waveguide and a solid-state microwave generator capable of applying microwaves to a fixed catalyst bed with spatially separated electric (E-) and magnetic (H-) fields and showed that the microwave flow reaction under the E-field improves the glucose formation rate up to 21.7 mmol/g per hour, which is 35.3 times higher than that achieved in the batch microwave reactor. AC-SO3H showed 16–30 times higher activity than Amberlyst 70 because of the higher dielectric loss tangent (tan δ) value of AC-SO3H (0.187) than Amberlyst 70 (0.040). H-field heating of AC-SO3H also improved the glucose formation rate by 1.2–1.6 times. Notably, the H-field reduced the microwave power to 45% of that of the E-field. Therefore, a microwave H-field flow reactor equipped with an AC-SO3H catalyst greatly improves both the glucose production rate and energy efficiency of cellobiose hydrolysis.
中文翻译:

在空间分离的微波电场和磁场流动反应器中通过磺化碳催化剂进行高效的纤维二糖水解
增强型多糖水解对于将多糖转化为单糖和低聚糖以用于食品、制药和生物基化学应用至关重要。在这项研究中,我们通过使用纤维二糖作为模型糖底物,通过应用微波和磺化碳催化剂 (AC-SO3H) 开发了一种高效的连续流水解工艺。我们建造了一个微波流动反应器,配备了矩形波导和固态微波发生器,能够将微波施加到具有空间分离的电场 (E-) 和磁场 (H-) 的固定催化剂床上,并表明电场下的微波流动反应将葡萄糖形成速率提高到每小时 21.7 mmol/g, 这比间歇式微波反应器所达到的水平高 35.3 倍。AC-SO3H 的活性比 Amberlyst 70 高 16-30 倍,因为 AC-SO3H 的介电损耗角正切 (tan δ) 值 (0.187) 高于 Amberlyst 70 (0.040)。AC-SO3H 的 H 场加热也使葡萄糖形成速率提高了 1.2-1.6 倍。值得注意的是,H 场将微波功率降低到电场的 45%。因此,配备 AC-SO3H 催化剂的微波 H 场流动反应器大大提高了纤维二糖水解的葡萄糖生成速率和能源效率。
更新日期:2024-12-16
中文翻译:

在空间分离的微波电场和磁场流动反应器中通过磺化碳催化剂进行高效的纤维二糖水解
增强型多糖水解对于将多糖转化为单糖和低聚糖以用于食品、制药和生物基化学应用至关重要。在这项研究中,我们通过使用纤维二糖作为模型糖底物,通过应用微波和磺化碳催化剂 (AC-SO3H) 开发了一种高效的连续流水解工艺。我们建造了一个微波流动反应器,配备了矩形波导和固态微波发生器,能够将微波施加到具有空间分离的电场 (E-) 和磁场 (H-) 的固定催化剂床上,并表明电场下的微波流动反应将葡萄糖形成速率提高到每小时 21.7 mmol/g, 这比间歇式微波反应器所达到的水平高 35.3 倍。AC-SO3H 的活性比 Amberlyst 70 高 16-30 倍,因为 AC-SO3H 的介电损耗角正切 (tan δ) 值 (0.187) 高于 Amberlyst 70 (0.040)。AC-SO3H 的 H 场加热也使葡萄糖形成速率提高了 1.2-1.6 倍。值得注意的是,H 场将微波功率降低到电场的 45%。因此,配备 AC-SO3H 催化剂的微波 H 场流动反应器大大提高了纤维二糖水解的葡萄糖生成速率和能源效率。































 京公网安备 11010802027423号
京公网安备 11010802027423号