Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In Vivo Surface-Enhanced Transmission Raman Spectroscopy and Impact of Frozen Biological Tissues on Lesion Depth Prediction
ACS Nano ( IF 15.8 ) Pub Date : 2024-12-16 , DOI: 10.1021/acsnano.4c12469 Yutong Zhou, Yumin Zhang, Haoqiang Xie, Zongyu Wu, Bowen Shi, Linley Li Lin, Jian Ye
ACS Nano ( IF 15.8 ) Pub Date : 2024-12-16 , DOI: 10.1021/acsnano.4c12469 Yutong Zhou, Yumin Zhang, Haoqiang Xie, Zongyu Wu, Bowen Shi, Linley Li Lin, Jian Ye
|
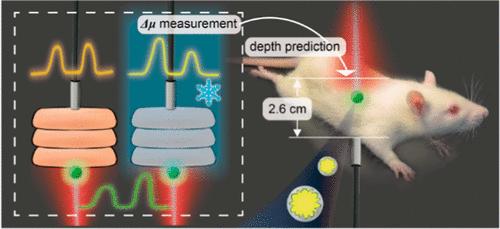
Plasmonic surface-enhanced transmission Raman spectroscopy (SETRS) has emerged as a promising optical technique for detecting and predicting the depths of deep-seated lesions in biological tissues. However, in vivo studies using SETRS are scarce and typically show shallow penetration depths. Moreover, the optical parameters used in the prediction process are often derived from frozen samples and there is limited understanding of how freezing affects the optical properties of biological tissues and the accuracy of depth prediction in living models. In this work, we conduct in vivo SETRS measurements on thick abdominal tissue region of the live rats to investigate the impact of freezing on the measured optical properties for the purpose of depth prediction. First, we fabricated ultrahigh bright surface-enhanced Raman spectroscopy (SERS) nanotags and utilized a custom transmission Raman system. We then measured the change of optical attenuation at two different wavelengths (Δμ) for four types of rat tissues (including skin, fat, muscle, and liver) following freezing. The freezing process dramatically affects Δμ values, even after only 1 day of freezing. In contrast, Δμ values obtained from fresh samples enable precise localization of SERS lesion phantoms in the live rat with only 5% deviation. The total thickness of the live rat is 2.6 cm, which, to the best of our knowledge, is the highest value of in vivo SETRS studies so far. This work helps to fill the gap in the SERS field of tissue localization and optical coefficient studies in highly heterogeneous tissues, and demonstrates the potential of the SETRS technique to achieve precise clinical localization of deep lesions.
中文翻译:

体内表面增强透射拉曼光谱和冷冻生物组织对病变深度预测的影响
等离子体表面增强透射拉曼光谱 (SETRS) 已成为一种很有前途的光学技术,用于检测和预测生物组织中深部病变的深度。然而,使用 SETRS 的体内研究很少,并且通常显示穿透深度较浅。此外,预测过程中使用的光学参数通常来自冷冻样品,对于冷冻如何影响生物组织的光学特性和活体模型中深度预测的准确性,人们的了解有限。在这项工作中,我们对活大鼠的厚腹部组织区域进行体内 SETRS 测量,以研究冻结对测量的光学特性的影响,以达到深度预测的目的。首先,我们制造了超高亮面增强拉曼光谱 (SERS) 纳米标签,并利用定制的透射拉曼系统。然后,我们测量了冷冻后四种大鼠组织 (包括皮肤、脂肪、肌肉和肝脏) 在两种不同波长 (Δμ) 下的光衰减变化。冷冻过程会显著影响 Δμ 值,即使仅冷冻 1 天后也是如此。相比之下,从新鲜样品中获得的 Δμ 值能够在活大鼠中精确定位 SERS 病变模型,偏差仅为 5%。活大鼠的总厚度为 2.6 厘米,据我们所知,这是迄今为止体内 SETRS 研究的最高值。这项工作有助于填补 SERS 领域在高度异质组织中组织定位和光学系数研究的空白,并展示了 SETRS 技术实现深部病灶精确临床定位的潜力。
更新日期:2024-12-17
中文翻译:

体内表面增强透射拉曼光谱和冷冻生物组织对病变深度预测的影响
等离子体表面增强透射拉曼光谱 (SETRS) 已成为一种很有前途的光学技术,用于检测和预测生物组织中深部病变的深度。然而,使用 SETRS 的体内研究很少,并且通常显示穿透深度较浅。此外,预测过程中使用的光学参数通常来自冷冻样品,对于冷冻如何影响生物组织的光学特性和活体模型中深度预测的准确性,人们的了解有限。在这项工作中,我们对活大鼠的厚腹部组织区域进行体内 SETRS 测量,以研究冻结对测量的光学特性的影响,以达到深度预测的目的。首先,我们制造了超高亮面增强拉曼光谱 (SERS) 纳米标签,并利用定制的透射拉曼系统。然后,我们测量了冷冻后四种大鼠组织 (包括皮肤、脂肪、肌肉和肝脏) 在两种不同波长 (Δμ) 下的光衰减变化。冷冻过程会显著影响 Δμ 值,即使仅冷冻 1 天后也是如此。相比之下,从新鲜样品中获得的 Δμ 值能够在活大鼠中精确定位 SERS 病变模型,偏差仅为 5%。活大鼠的总厚度为 2.6 厘米,据我们所知,这是迄今为止体内 SETRS 研究的最高值。这项工作有助于填补 SERS 领域在高度异质组织中组织定位和光学系数研究的空白,并展示了 SETRS 技术实现深部病灶精确临床定位的潜力。






























 京公网安备 11010802027423号
京公网安备 11010802027423号