当前位置:
X-MOL 学术
›
Appl. Surf. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Physical and electronic structure optimization of multivalent multi-dimensional Cu-based electrodes for efficient electrocatalytic nitrate reduction to ammonia
Applied Surface Science ( IF 6.3 ) Pub Date : 2024-12-16 , DOI: 10.1016/j.apsusc.2024.162078 Xiaojing Yu, Kaiyuan Li, Fuping Li, Bin Wang, Shaodong Sun, Yufei Tang, Zhipeng Li, Kang Zhao
Applied Surface Science ( IF 6.3 ) Pub Date : 2024-12-16 , DOI: 10.1016/j.apsusc.2024.162078 Xiaojing Yu, Kaiyuan Li, Fuping Li, Bin Wang, Shaodong Sun, Yufei Tang, Zhipeng Li, Kang Zhao
|
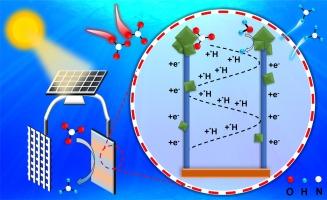
The electrochemical reduction of nitrate for ammonia synthesis has attracted considerable attention due to its low energy consumption and environmental compatibility. To facilitate the industrial-scale implementation of catalysts for electrochemical ammonia production, it is crucial to consider not only the catalysts’ high catalytic activity and selectivity but also their scalable fabrication process and facile preparation methodology. This study presented a multi-dimensional composite electrode with multivalent Cu-based oxides designed using a simple immersion reduction method. Cu(OH)2 nanowires and Cu2O nanoparticles were in-situ grown on Cu foam, creating a multidimensional composite structure. Subsequently, the electrode is transformed into Cu+/Cu0 through electrochemical in-situ reduction, while the microstructure and morphology do not undergo significant changes. The electronic interactions between multivalent Cu-based oxides promoted physicochemical adsorption of NO3– molecules and optimize electron and proton transfer pathways. At a potential of −0.8 V (vs. RHE) in neutral electrolyte, the multivalent Cu-based electrode achieved the nitrate conversion of 99.99 %, NH3 yield rate of 1040.82 µg h−1 cm−2 and NH3 Selectivity of 99.5 %. Furthermore, the electrodes demonstrated high nitrate conversion and good NH3 yield when powered by a small solar photovoltaic panel, suggesting potential for industrial-scale production using renewable energy sources.
中文翻译:

多价多维铜基电极的物理和电子结构优化,用于高效电催化硝酸盐还原成氨
硝酸盐电化学还原用于合成氨因其低能耗和环境兼容性而受到广泛关注。为了促进用于电化学氨生产的催化剂的工业规模实施,不仅要考虑催化剂的高催化活性和选择性,还要考虑其可扩展的制造工艺和简单的制备方法,这一点至关重要。本研究提出了一种使用简单的浸入还原法设计的具有多价 Cu 基氧化物的多维复合电极。Cu(OH) 2 纳米线和 Cu 2 O 纳米颗粒在泡沫铜上原位生长,形成多维复合结构。随后, 0 电极通过电化学原位还原转化为 Cu + /Cu,而微观结构和形貌没有发生显着变化。多价 Cu 基氧化物之间的电子相互作用促进了 NO 3 – 分子的物理化学吸附,并优化了电子和质子转移途径。在中性电解质中 −0.8 V(相对于 RHE)的电位下,多价铜基电极实现了 99.99% 的硝酸盐转化率、1040.82 μg h −1 cm −2 的 NH 3 产率和 99.5% 的 NH 3 选择性。此外,当由小型太阳能光伏板供电时,电极表现出高硝酸盐转化率和良好的 NH 3 产率,这表明具有使用可再生能源进行工业规模生产的潜力。
更新日期:2024-12-18
中文翻译:

多价多维铜基电极的物理和电子结构优化,用于高效电催化硝酸盐还原成氨
硝酸盐电化学还原用于合成氨因其低能耗和环境兼容性而受到广泛关注。为了促进用于电化学氨生产的催化剂的工业规模实施,不仅要考虑催化剂的高催化活性和选择性,还要考虑其可扩展的制造工艺和简单的制备方法,这一点至关重要。本研究提出了一种使用简单的浸入还原法设计的具有多价 Cu 基氧化物的多维复合电极。Cu(OH) 2 纳米线和 Cu 2 O 纳米颗粒在泡沫铜上原位生长,形成多维复合结构。随后, 0 电极通过电化学原位还原转化为 Cu + /Cu,而微观结构和形貌没有发生显着变化。多价 Cu 基氧化物之间的电子相互作用促进了 NO 3 – 分子的物理化学吸附,并优化了电子和质子转移途径。在中性电解质中 −0.8 V(相对于 RHE)的电位下,多价铜基电极实现了 99.99% 的硝酸盐转化率、1040.82 μg h −1 cm −2 的 NH 3 产率和 99.5% 的 NH 3 选择性。此外,当由小型太阳能光伏板供电时,电极表现出高硝酸盐转化率和良好的 NH 3 产率,这表明具有使用可再生能源进行工业规模生产的潜力。






























 京公网安备 11010802027423号
京公网安备 11010802027423号