当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery and biological evaluation of potent 2-trifluoromethyl acrylamide warhead-containing inhibitors of protein disulfide isomerase
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2024-12-16 , DOI: 10.1016/j.ejmech.2024.117169 Jung-Chun Chu, Keng-Chang Tsai, Ting-Yu Wang, Tzu-Yin Chen, Ju-Ying Tsai, Tien Lee, Mei-Hsiang Lin, Yves S.Y. Hsieh, Chin-Chung Wu, Wei-Jan Huang
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2024-12-16 , DOI: 10.1016/j.ejmech.2024.117169 Jung-Chun Chu, Keng-Chang Tsai, Ting-Yu Wang, Tzu-Yin Chen, Ju-Ying Tsai, Tien Lee, Mei-Hsiang Lin, Yves S.Y. Hsieh, Chin-Chung Wu, Wei-Jan Huang
|
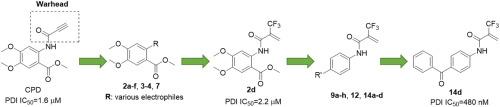
Protein disulfide isomerase (PDI) regulates multiple protein functions by catalyzing the oxidation, reduction, and isomerization of disulfide bonds. The enzyme is considered a potential target for treating thrombosis. We previously developed a potent PDI inhibitor, CPD, which contains the propiolamide as a warhead targeting cysteine residue in PDI. To address its issues with undesirable off-target effects and weak metabolic stability, we replaced the propiolamide group with various electrophiles. Among these, compound 2d, which contains 2-trifluoromethyl acrylamide exhibited potent PDI inhibition compared to the reference PACMA31. Further structural optimization of compound 2d led to a novel series of 2-trifluoromethyl acrylamide derivatives. Several of these compounds displayed substantially improved enzyme inhibition. Notably, compound 14d demonstrated the strongest inhibition against PDI, with an IC50 value of 0.48 ± 0.004 μM. Additionally, compound 14d was found to exhibit a reversible binding mode with PDI enzyme. Further biological evaluations show that 14d suppressed platelet aggregation and thrombus formation by attenuating GPIIb/IIIa activation without significantly causing cytotoxicity. Altogether, these findings suggest PDI inhibitors could be a potential strategy for anti-thrombosis.
中文翻译:

强效 2-三氟甲基丙烯酰胺弹头蛋白二硫键异构酶抑制剂的发现和生物学评价
蛋白质二硫键异构酶 (PDI) 通过催化二硫键的氧化、还原和异构化来调节多种蛋白质功能。这种酶被认为是治疗血栓形成的潜在靶点。我们之前开发了一种有效的 PDI 抑制剂 CPD,它包含丙噻酰胺作为靶向 PDI 中半胱氨酸残基的弹头。为了解决其不良脱靶效应和较弱的代谢稳定性的问题,我们用各种亲电试剂取代了丙硫酰胺基团。其中,与参比 2-三氟甲基丙烯酰胺相比,含有 2-三氟甲基丙烯酰胺的化合物 2d 表现出有效的 PDI 抑制PACMA31。化合物 2d 的进一步结构优化导致了一系列新颖的 2-三氟甲基丙烯酰胺衍生物。其中一些化合物显示出显着改善的酶抑制。值得注意的是,化合物 14d 对 PDI 的抑制作用最强,IC50 值为 0.48 ± 0.004 μM。此外,发现化合物 14d 与 PDI 酶表现出可逆结合模式。进一步的生物学评估表明,14d 通过减弱 GPIIb/IIIa 活化来抑制血小板聚集和血栓形成,而不会显着引起细胞毒性。总而言之,这些发现表明 PDI 抑制剂可能是一种潜在的抗血栓形成策略。
更新日期:2024-12-20
中文翻译:

强效 2-三氟甲基丙烯酰胺弹头蛋白二硫键异构酶抑制剂的发现和生物学评价
蛋白质二硫键异构酶 (PDI) 通过催化二硫键的氧化、还原和异构化来调节多种蛋白质功能。这种酶被认为是治疗血栓形成的潜在靶点。我们之前开发了一种有效的 PDI 抑制剂 CPD,它包含丙噻酰胺作为靶向 PDI 中半胱氨酸残基的弹头。为了解决其不良脱靶效应和较弱的代谢稳定性的问题,我们用各种亲电试剂取代了丙硫酰胺基团。其中,与参比 2-三氟甲基丙烯酰胺相比,含有 2-三氟甲基丙烯酰胺的化合物 2d 表现出有效的 PDI 抑制PACMA31。化合物 2d 的进一步结构优化导致了一系列新颖的 2-三氟甲基丙烯酰胺衍生物。其中一些化合物显示出显着改善的酶抑制。值得注意的是,化合物 14d 对 PDI 的抑制作用最强,IC50 值为 0.48 ± 0.004 μM。此外,发现化合物 14d 与 PDI 酶表现出可逆结合模式。进一步的生物学评估表明,14d 通过减弱 GPIIb/IIIa 活化来抑制血小板聚集和血栓形成,而不会显着引起细胞毒性。总而言之,这些发现表明 PDI 抑制剂可能是一种潜在的抗血栓形成策略。






























 京公网安备 11010802027423号
京公网安备 11010802027423号