当前位置:
X-MOL 学术
›
ACS Energy Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Detail Matters: Unveiling Overlooked Parameters in the Mechanochemical Synthesis of Solid Electrolytes
ACS Energy Letters ( IF 19.3 ) Pub Date : 2024-12-16 , DOI: 10.1021/acsenergylett.4c02156 Abdulkadir Kızılaslan, Mustafa Çelik, Yuta Fujii, Zheng Huang, Chikako Moriyoshi, Shogo Kawaguchi, Satoshi Hiroi, Koji Ohara, Mariko Ando, Kiyoharu Tadanaga, Saneyuki Ohno, Akira Miura
ACS Energy Letters ( IF 19.3 ) Pub Date : 2024-12-16 , DOI: 10.1021/acsenergylett.4c02156 Abdulkadir Kızılaslan, Mustafa Çelik, Yuta Fujii, Zheng Huang, Chikako Moriyoshi, Shogo Kawaguchi, Satoshi Hiroi, Koji Ohara, Mariko Ando, Kiyoharu Tadanaga, Saneyuki Ohno, Akira Miura
|
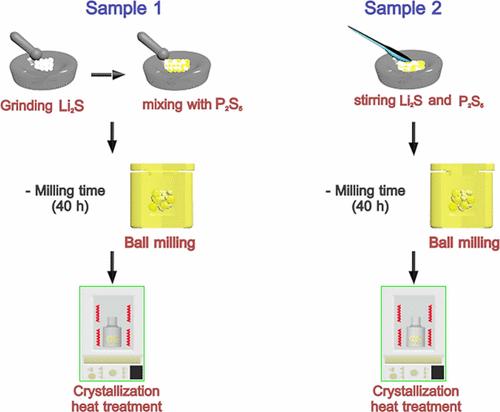
The advent of all-solid-state lithium-ion batteries has advanced energy storage technologies with the development of highly conductive solid electrolytes. Numerous researchers have reported the structural and electrochemical performance of solid electrolytes obtained through different production techniques and with different compositions. (1,2) However, even in relatively robust production techniques using ball-milling with the same composition and stoichiometry, only a minute difference in the synthesis process can significantly affect the crystallization mechanisms and resulting ionic conductivity, thereby highlighting the importance of overlooked parameters. This Viewpoint demonstrates the effects of “premixing”─mixing the precursors with a mortar and pestle prior to the mechanochemical synthesis of glassy solid electrolytes, particularly Li2S–P2S5 sulfides and the newly emerging NaTaCl6 halides─on the structure and transport of the resulting products. Crystal structures and amorphous configurations of sulfide and chloride electrolytes with high ionic conductivities and excellent mechanical properties have been identified. (3−11) These electrolytes are commonly produced through mechanochemical synthesis using ball-milling, which has been widely utilized with various chemical compounds. (12−14) Li7P3S11 is recognized as a metastable phase that is nucleated by the subsequent heat treatment of Li2S–P2S5 glasses produced by planetary ball-milling. NaTaCl6 is recognized as a mixture of crystal and amorphous phases that shows an excellent electrochemical window. In both cases, a wide range of ball-milling experimental parameters have been investigated, including the amount of powder, number of balls, rotation speed, and ball-milling time, leading to the successful synthesis of the target phases. (15−24) This experimental fact makes these synthesis methods for producing Li7P3S11 and NaTaCl6 seemingly very robust. Nonetheless, although the apparent crystal structures evaluated by X-ray diffraction (XRD) are almost identical, there are significant differences in their ion conductivities. (18,25−27) Moreover, only three of 15 studies reported on Li7P3S11 syntheses (3,28,29) have described the hand-mixing of starting powders prior to or intermittently during ball-milling, as given in Table S1. Similarly, one out of three studies of NaTaCl6 have described the hand-mixing of starting powders (Table S2). Although the details of the hand-mixing have been deemed to have negligible effects, this study demonstrates the importance of such a process. This Viewpoint demonstrates the impact of the unspecified details of mechanochemical synthesis on the crystallization mechanisms and ionic conductivities of the products, highlighting the importance of parameters that are often overlooked in such syntheses. Two synthesis routes based on the existing literature (3,6,17,30) were employed to synthesize Li7P3S11 using ball-milling, as shown in Scheme 1. The difference between the two routes is the implementation of a hand-mixing step prior to mechanical milling, referred to as premixing. For Sample 1, the hand-ground Li2S precursor was thoroughly mixed together with P2S5 for 20 min using an agate mortar and pestle. In contrast, the precursors of Sample 2 were just stirred with a spatula for 2 min prior to milling. Similarly, the impact of premixing on the synthesis of NaTaCl6, synthesized by mechanical milling of NaCl and TaCl5, was assessed through investigating two NaTaCl6 samples synthesized with or without premixing. The details of the compositions during the synthesis of the powders, including the required reagents and synthesis process, are given in Tables S3 and S4. Figure 1(a, b) displays the 31P magic-angle spinning nuclear magnetic resonance (31P MAS NMR) spectra of the Li7P3S11 glass produced by milling with or without premixing before heating. Despite the broad halo patterns in XRD (Figure S1), which indicate that both the samples, with and without premixing, were completely amorphous, distinctly different local anion coordination is observed in the NMR spectra. The spectra of both samples were deconvoluted into three peaks, where PS43– and P2S74– are constituent elements of the target Li7P3S11 phase and P2S64– is the local unit of the Li4P2S6 impurity phase. (19) Figure 1(c) shows a comparison of the area-size fractions of the peaks corresponding to the three anion blocks. Although a glassy phase typically contains various local structures as its nature, the amount of P2S64– in Sample 1 is less than that in Sample 2. Furthermore, the area-size ratios of P2S74–, and PS43– are 1.70 and 1.24 for Samples 1 and 2, respectively. The area-size ratio of Sample 1 approaches 2.00, which is the theoretical stoichiometric ratio of the two components in crystalline Li7P3S11, indicating the anion blocks in Sample 1 are similar to those in Li7P3S11 even before heat treatment. Data with a wider range of chemical shifts, confirming no overlap between sidebands and main peaks, are shown in Figure S2. Figure 1. 31P MAS NMR spectra and deconvoluted peak profiles of the as-milled powders of (a) Sample 1 and (b) Sample 2. (c) Area-size fractions of Samples 1 and 2. In situ synchrotron X-ray diffraction (SXRD) demonstrates evidently different crystallization processes between the two Li2S–P2S5 glasses (Figure 2). Sample 1 exhibits a single-step crystallization at approximately 220 °C. Conversely, Sample 2 undergoes a two-step crystallization process owing to the sequential formation of Li3PS4 at approximately 220 °C, followed by Li7P3S11 at approximately 240 °C. Rietveld refinement was performed on the temperature-dependent diffractograms to quantify the Li7P3S11, Li3PS4, and Li4P2S6 fractions at various temperatures. Sample 1 crystallizes into Li7P3S11 without apparent side phases, whereas Sample 2 comprises 11% Li3PS4 and 2% Li4P2S6 even at 300 °C, which can degrade its ion-transport properties. (30−33) The diffraction profiles did not show a significant difference in the peaks of the Li7P3S11 phase (Figure S3). Differential thermal analysis (DTA) confirmed the difference in the crystallization processes (Figure S4). While a sharp exothermic signal was observed in Sample 1, a broad signal with two distinct peaks was seen in Sample 2. This trend was reproducible even for the same samples in different batches, indicating the local inhomogeneity of the anion blocks in the original glass states, as revealed in the NMR data. Figure 2. SXRD data of the powders heated at 60 °C/min under a N2 flow and phase ratios from Rietveld refinement of (a) Sample 1 and (b) Sample 2. A distinctly different crystallization process induced by local structural differences leads to variations in the properties of the resulting crystalline Li7P3S11 phases. Figure S5 shows the 31P NMR spectra of the two samples after crystallization. While the quantification of local units is challenging with the spectra from the heat-treated samples due to the appearance of the cross peaks in Sample 1, the qualitative difference is clearly observed. It should be noted that such cross peaks associated with P2S74– and PS43– are commonly observed in well-crystalline Li7P3S11 phases. (34) To further investigate the differences in the crystalline phases of Samples 1 and 2, pair-distribution function (PDF) analysis was performed on Samples 1 and 2. As the data from both samples showed the appearance of long-range ordering only after heating, the two samples were largely similar. However, a slight change in G(r) in short-range order of ∼3.4 Å appeared, depending on the hand-milling processes (Figure S6). The morphologies of Samples 1 and 2 after heating showed no significant differences (Figure S7). Overall, it is evident that the premixing procedure prior to milling severely impacts the resulting structure. The above-mentioned differences led to significant differences in the ion-transport properties of the resulting materials. The powders crystallized at 280–300 °C were pressed into pellets, and their ionic conductivities were measured by temperature-dependent impedance spectroscopy to analyze the Li-ion transport in the samples. Furthermore, the migration barrier of the Li-ion conduction was determined according to the Arrhenius relation: Figure 3. Arrhenius plots of the temperature and ion conductivity of Samples 1 and 2. As another model system to assess the impact of premixing, we employed a newly found halide-based solid electrolyte, NaTaCl6, to further investigate the impact of premixing. The ion-transport properties of NaTaCl6 are reported to significantly vary with its crystallinity. (27) Moreover, the impact of elongated milling time of mechanochemical synthesis on the ion-transport properties has been revealed. (26) In this section, four samples were synthesized, either with or without premixing, and subjected to milling for either 16.5 or 100 h. Figure 4 shows the room-temperature ionic conductivity and activation energies of four samples obtained with different milling times, with or without premixing. The samples without premixing were synthesized via ball-milling; that is, the precursors were directly placed into the ball-mill cup with the milling media. Meanwhile, the samples with premixing were mixed well with a mortar and pestle before ball-milling. Prolonging the milling time from 16.5 to 100 h slightly improved the ionic conductivity. However, more significant improvements in the ionic conductivity were achieved by premixing the precursors well, whereas there is no significant difference in their XRD patterns. Notably, milling for 100 h was insufficient to achieve the same level of ion transport as that of the sample subjected to premixing. These results highlight the importance of the premixing step. Figure 4. Ionic conductivity and activation energies of mechanochemically synthesized NaTaCl6 samples under different conditions. Each sample was synthesized three times, and the error bars are the standard deviations from the three batches of samples. Li7P3S11 and NaTaCl6 syntheses highlight the crucial effect of the hand-mixing step before mechanical milling on the local structure, crystallization temperature, and ionic conductivity. Although further accumulation of experimental evidence is necessary to precisely elucidate the underlying mechanisms, the current findings are as follows: Significant variations in the fractions of the local units, indicating the local inhomogeneity, were observed without the implementation of the premixing step. These localized compositional variations can lead to diverse anionic blocks during the subsequent crystallization processes, which, in turn, alters the structure and properties of the products. In the case of NaTaCl6, extended ball-milling durations did not eliminate localized compositional variations, as inferred from the limited conductivity improvement when the ball-milling time was increased from 16.5 to 100 h. Meanwhile, the resulting ion transport was greatly improved by the introduction of a short premixing step before mechanochemical synthesis. While the impact of extended milling may vary with the chemical and mechanical properties of the precursors, the premixing procedure helps to reduce the synthesis time to obtain the target phases. Differential adhesion to the milling media was proposed as a potential cause of compositional inhomogeneity. Ball-milling homogenized mixed precursors when they were trapped between the milling media or between the media and the inner wall of the cup. This process formed powders with layered structures of various compositions. Such a reaction was promoted by mechanical mixing as the interlayer distance decreased, reducing the diffusion length required to form the target phase. However, when a heterogeneous precursor mixture was introduced into the milling cup, softer materials tended to adhere to the milling medium first. Consequently, we hypothesized that this formed thick layers with a constant diffusion length, leading to pronounced local compositional variations. Mechanical properties of starting materials and final products can be critical for proceeding the reactions. (35) As significant variation in the seemingly reproducible properties─ionic conductivity and cycling performance─is one of the big challenges in the field of solid-state batteries and attracts increasing attention, with the recent reports showcasing the critical issue, (36,37) our findings on the drastic impact of the premixing procedure before the seemingly robust mechanochemical synthesis with ball-milling further highlight the importance of the details in the synthesis conditions. The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsenergylett.4c02156. Table of the synthesis process of Li7P3S11 and NaTaCl6, characterization details, measurement results (XRD patterns, NMR spectra, DTA curves, PDF and SEM images) of Li7P3S11 (PDF) The Detail Matters: Unveiling Overlooked Parameters
in the Mechanochemical Synthesis of Solid Electrolytes 1 views 0 shares 0 downloads Most electronic Supporting Information files are available without a subscription to ACS Web Editions. Such files may be downloaded by article for research use (if there is a public use license linked to the relevant article, that license may permit other uses). Permission may be obtained from ACS for other uses through requests via the RightsLink permission system: http://pubs.acs.org/page/copyright/permissions.html. A. Miura is grateful to Ms. Masae Sawamoto for preparing the powder capillaries for the SXRD measurements. The authors are grateful to Mr. Yuki Chiba at Tohoku University for his support with the solid-state MAS NMR measurements. S.O. gratefully acknowledges Toyota Riken for its financial support through the Rising Fellows Program. This study was partially supported by JST PRESTO JPMJPR21Q8, JST Gtex JPMJGX23S5, and JPMJGX23S2. This article references 37 other publications. This article has not yet been cited by other publications.
中文翻译:

细节很重要:揭示固体电解质机械化学合成中被忽视的参数
全固态锂离子电池的出现随着高导电性固体电解质的发展,具有先进的储能技术。许多研究人员报道了通过不同生产技术和不同成分获得的固体电解质的结构和电化学性能。(1,2) 然而,即使在使用具有相同成分和化学计量的球磨的相对稳健的生产技术中,合成过程中的微小差异也会显着影响结晶机制和由此产生的离子电导率,从而突出了被忽视参数的重要性。该观点展示了“预混合”的影响,即在机械化学合成玻璃状固体电解质(特别是 Li2S-P2S5 硫化物和新出现的 NaTaCl6 卤化物)之前,将前驱体与研钵和研杵混合,对所得产物的结构和传输的影响。已经确定了具有高离子电导率和优异机械性能的硫化物和氯离子电解质的晶体结构和无定形构型。(3−11) 这些电解质通常是通过使用球磨法进行机械化学合成生产的,球磨法已广泛用于各种化合物。(12−14) Li7P3S11 被认为是一种亚稳相,通过行星球磨生产的 Li2S–P2S5 玻璃的后续热处理而成核。NaTaCl6 被认为是晶相和非晶相的混合物,显示出优异的电化学窗口。 在这两种情况下,都研究了广泛的球磨实验参数,包括粉末量、球数、转速和球磨时间,从而成功合成了目标相。(15−24) 这一实验事实使得这些生产 Li7P3S11 和 NaTaCl6 的合成方法似乎非常稳健。尽管如此,尽管通过 X 射线衍射 (XRD) 评估的表观晶体结构几乎相同,但它们的离子电导率存在显着差异。(18,25−27) 此外,在报告 Li7P3S11 合成的 15 项研究中,只有 3 项 (3,28,29) 描述了在球磨之前或期间间歇性地手动混合起始粉末,如表 S1 所示。同样,对 NaTaCl6 的三项研究中就有一研究描述了起始粉末的手工混合(表 S2)。尽管手工混合的细节被认为影响可以忽略不计,但这项研究证明了这种过程的重要性。该观点展示了机械化学合成的未指定细节对产物结晶机制和离子电导率的影响,强调了在此类合成中经常被忽视的参数的重要性。基于现有文献 (3,6,17,30) 的两条合成路线采用球磨法合成 Li7P3S11,如图 1 所示。这两种路线的区别在于在机械研磨之前实施手动混合步骤,称为预混合。 对于样品 1,使用玛瑙研钵和研杵将手磨的 Li2S 前驱体与 P2S5 充分混合 20 分钟。相比之下,样品 2 的母离子在研磨前仅用刮刀搅拌 2 min。同样,通过研究两个有或没有预混合合成的 NaTaCl6 样品,评估了预混合对 NaTaCl 6 合成的影响,NaTaCl 6 通过机械研磨 NaCl和 TaCl5 合成。表 S3 和 S4 中给出了粉末合成过程中组合物的详细信息,包括所需的试剂和合成过程。图 1(a, b) 显示了 Li7P3S11 玻璃的 31P 魔角旋转核磁共振 (31P MAS NMR) 光谱,该光谱是在加热前有或没有预混合的情况下进行铣削生产的。尽管 XRD 中存在较宽的光晕图案(图 S1),这表明有预混和无预混的样品都是完全无定形的,但在 NMR 波谱中观察到明显不同的局部阴离子配位。将两个样品的光谱解卷积为三个峰,其中 PS43– 和 P2S74– 是目标 Li7P3S11 相的组成元素,P2S64– 是 Li4P2S6 杂质相的局部单位。(19) 图 1(c) 显示了对应于三个阴离子块的峰的峰的面积大小分数的比较。尽管玻璃相的性质通常包含各种局部结构,但样品 1 中 P2S 6 4– 的量少于样品 2 中的 P 2 S64– 量。 此外,样品 1 和 2 的 P2S74– 和 PS43– 的面积大小比分别为 1.70 和 1.24。样品 1 的面积尺寸比接近 2.00,这是结晶 Li7P3S11 中两种组分的理论化学计量比,表明样品 1 中的阴离子块与热处理前的 Li7P3S11 中的阴离子块相似。图 S2 显示了具有更大范围化学位移的数据,证实边带和主峰之间没有重叠。图 1.31(a) 样品 1 和 (b) 样品 2 的研磨粉末的 P MAS NMR 谱图和去卷积峰剖面。(c) 样品 1 和 2 的面积大小分数。原位同步加速器 X 射线衍射 (SXRD) 表明两种 Li2S-P2S5 玻璃之间的结晶过程明显不同(图 2)。样品 1 在大约 220 °C 时表现出单步结晶。 相反,样品 2 在大约 220 °C 时连续形成 Li3PS4,然后在大约 240 °C 下依次形成 Li7P3S11,因此经历了两步结晶过程。 对温度依赖性衍射图进行 Rietveld 精修,以量化不同温度下的 Li7P3S11、Li3PS4 和 Li4P2S6 馏分。样品 1 结晶成 Li7P3S11,没有明显的副相,而样品 2 即使在 300 °C 下也含有 11% 的 Li3PS4 和 2% 的 Li4P2S6,这会降低其离子传输性能。 (30−33) 衍射曲线未显示 Li7P3S11 相的峰存在显著差异(图 S3)。差热分析 (DTA) 证实了结晶过程的差异(图 S4)。虽然在样品 1 中观察到尖锐的放热信号,但在样品 2 中观察到具有两个不同峰的宽信号。即使对于不同批次的相同样品,这种趋势也是可重现的,这表明阴离子块在原始玻璃状态下的局部不均匀性,如 NMR 数据所示。图 2.在 N2 流速和相比下以 60 °C/min 加热的粉末的 SXRD 数据,来自 Rietveld 精炼的 (a) 样品 1 和 (b) 样品 2。由局部结构差异引起的明显不同的结晶过程导致所得结晶 Li7P3S11 相的性质发生变化。图 S5 显示了结晶后两个样品的 31P NMR 波谱。虽然由于样品 1 中出现交叉峰,热处理样品的光谱难以定量局部单位,但可以清楚地观察到定性差异。应该注意的是,这种与 P2S74– 和 PS43– 相关的交叉峰通常出现在结晶良好的 Li7P3S11 相中。(34) 为了进一步研究样品 1 和 2 的晶相差异,对样品 1 和 2 进行了对分布函数 (PDF) 分析。由于两个样品的数据仅在加热后才出现长程排序,因此两个样品基本相似。 然而,根据手工铣削工艺的不同,G(r) 在短程 ∼3.4 Å 的量程中出现了轻微的变化(图 S6)。加热后样品 1 和 2 的形态没有显示显着差异(图 S7)。总体而言,很明显,铣削前的预混合程序会严重影响最终结构。上述差异导致所得材料的离子传输特性存在显著差异。将在 280–300 °C 下结晶的粉末压制成颗粒,并通过温度依赖性阻抗谱测量其离子电导率,以分析样品中的锂离子传输。此外,根据 Arrhenius 关系确定锂离子传导的迁移势垒:图 3.样品 1 和 2 的温度和离子电导率的 Arrhenius 图。作为评估预混合影响的另一种模型系统,我们采用了一种新发现的卤化物基固体电解质 NaTaCl6 来进一步研究预混合的影响。据报道,NaTaCl6 的离子传输特性随其结晶度而显着变化。(27) 此外,已经揭示了机械化学合成的拉长研磨时间对离子传输性能的影响。(26) 在本节中,合成了四个样品,有或没有预混合,并进行了 16.5 或 100 小时的研磨。图 4 显示了在不同研磨时间(有或没有预混合)下获得的四个样品的室温离子电导率和活化能。未预混合的样品通过球磨合成;也就是说,前驱体与研磨介质一起直接放入球磨杯中。 同时,将预混样品与研钵和研杵充分混合后,再进行球磨。将研磨时间从 16.5 h 延长到 100 h 略微提高了离子电导率。然而,通过充分预混合前驱体,离子电导率得到了更显著的改善,而它们的 XRD 图谱没有显著差异。值得注意的是,研磨 100 小时不足以达到与预混合样品相同的离子传输水平。这些结果突出了预混合步骤的重要性。图 4.机械化学合成的 NaTaCl6 样品在不同条件下的离子电导率和活化能。每个样品合成 3 次,误差线是 3 个批次样品的标准差。Li7P3S11 和 NaTaCl6 合成强调了机械研磨前手工混合步骤对局部结构、结晶温度和离子电导率的关键影响。尽管需要进一步积累实验证据以精确阐明潜在机制,但目前的发现如下:在没有实施预混合步骤的情况下观察到局部单位分数的显着变化,表明局部不均匀性。这些局部成分变化会导致在随后的结晶过程中产生不同的阴离子嵌段,进而改变产品的结构和特性。 在 NaTaCl6 的情况下,延长球磨持续时间并不能消除局部成分变化,这从球磨时间从 16.5 小时增加到 100 小时时电导率的有限改善可以推断出来。同时,通过在机械化学合成之前引入简短的预混步骤,所得的离子传输得到了极大的改善。虽然延长研磨的影响可能因前驱体的化学和机械性能而异,但预混合程序有助于缩短合成时间以获得目标相。对研磨介质的粘附力差异被认为是成分不均匀的潜在原因。球磨将混合前驱体均质化,当它们被困在研磨介质之间或介质与杯内壁之间时。这个过程形成了具有各种成分的层状结构的粉末。随着层间距离的减小,机械混合促进了这种反应,从而减少了形成目标相所需的扩散长度。然而,当异质前驱体混合物被引入研磨杯中时,较软的材料往往会首先粘附在研磨介质上。因此,我们假设这形成了具有恒定扩散长度的厚层,导致明显的局部成分变化。起始材料和最终产物的机械性能对于反应的进行至关重要。 (35) 由于看似可重复的性质(离子电导率和循环性能)的显着变化是固态电池领域的巨大挑战之一,并引起了越来越多的关注,最近的报告展示了关键问题,(36,37) 我们对在看似稳健的球磨机械化学合成之前预混合程序的巨大影响的研究结果进一步强调了合成条件中细节的重要性。支持信息可在 https://pubs.acs.org/doi/10.1021/acsenergylett.4c02156 免费获取。Li7P3S11 和 NaTaCl6 的合成过程表、表征细节、Li7P3S11 的测量结果(XRD 图谱、NMR 光谱、DTA 曲线、PDF 和 SEM 图像) (PDF)细节很重要:揭示固体电解质机械化学合成中被忽视的参数 1 次浏览 0 分享 0 下载 大多数电子支持信息文件无需订阅 ACS Web 版本即可获得。此类文件可以按文章下载用于研究用途(如果有链接到相关文章的公共使用许可证,则该许可证可能允许其他用途)。可以通过 RightsLink 权限系统(http://pubs.acs.org/page/copyright/permissions.html)请求,从 ACS 获得用于其他用途的权限。A. Miura 感谢 Masae Sawamoto 女士为 SXRD 测量准备粉末毛细管。作者感谢东北大学的 Yuki Chiba 先生对固体 MAS NMR 测量的支持。S.O. 衷心感谢丰田理研通过 Rising Fellows 计划提供的财政支持。本研究得到了 JST PRESTO JPMJPR21Q8 、 JST Gtex JPMJGX23S5 和 JPMJGX23S2 的部分支持。本文引用了其他 37 种出版物。本文尚未被其他出版物引用。
更新日期:2024-12-17
中文翻译:

细节很重要:揭示固体电解质机械化学合成中被忽视的参数
全固态锂离子电池的出现随着高导电性固体电解质的发展,具有先进的储能技术。许多研究人员报道了通过不同生产技术和不同成分获得的固体电解质的结构和电化学性能。(1,2) 然而,即使在使用具有相同成分和化学计量的球磨的相对稳健的生产技术中,合成过程中的微小差异也会显着影响结晶机制和由此产生的离子电导率,从而突出了被忽视参数的重要性。该观点展示了“预混合”的影响,即在机械化学合成玻璃状固体电解质(特别是 Li2S-P2S5 硫化物和新出现的 NaTaCl6 卤化物)之前,将前驱体与研钵和研杵混合,对所得产物的结构和传输的影响。已经确定了具有高离子电导率和优异机械性能的硫化物和氯离子电解质的晶体结构和无定形构型。(3−11) 这些电解质通常是通过使用球磨法进行机械化学合成生产的,球磨法已广泛用于各种化合物。(12−14) Li7P3S11 被认为是一种亚稳相,通过行星球磨生产的 Li2S–P2S5 玻璃的后续热处理而成核。NaTaCl6 被认为是晶相和非晶相的混合物,显示出优异的电化学窗口。 在这两种情况下,都研究了广泛的球磨实验参数,包括粉末量、球数、转速和球磨时间,从而成功合成了目标相。(15−24) 这一实验事实使得这些生产 Li7P3S11 和 NaTaCl6 的合成方法似乎非常稳健。尽管如此,尽管通过 X 射线衍射 (XRD) 评估的表观晶体结构几乎相同,但它们的离子电导率存在显着差异。(18,25−27) 此外,在报告 Li7P3S11 合成的 15 项研究中,只有 3 项 (3,28,29) 描述了在球磨之前或期间间歇性地手动混合起始粉末,如表 S1 所示。同样,对 NaTaCl6 的三项研究中就有一研究描述了起始粉末的手工混合(表 S2)。尽管手工混合的细节被认为影响可以忽略不计,但这项研究证明了这种过程的重要性。该观点展示了机械化学合成的未指定细节对产物结晶机制和离子电导率的影响,强调了在此类合成中经常被忽视的参数的重要性。基于现有文献 (3,6,17,30) 的两条合成路线采用球磨法合成 Li7P3S11,如图 1 所示。这两种路线的区别在于在机械研磨之前实施手动混合步骤,称为预混合。 对于样品 1,使用玛瑙研钵和研杵将手磨的 Li2S 前驱体与 P2S5 充分混合 20 分钟。相比之下,样品 2 的母离子在研磨前仅用刮刀搅拌 2 min。同样,通过研究两个有或没有预混合合成的 NaTaCl6 样品,评估了预混合对 NaTaCl 6 合成的影响,NaTaCl 6 通过机械研磨 NaCl和 TaCl5 合成。表 S3 和 S4 中给出了粉末合成过程中组合物的详细信息,包括所需的试剂和合成过程。图 1(a, b) 显示了 Li7P3S11 玻璃的 31P 魔角旋转核磁共振 (31P MAS NMR) 光谱,该光谱是在加热前有或没有预混合的情况下进行铣削生产的。尽管 XRD 中存在较宽的光晕图案(图 S1),这表明有预混和无预混的样品都是完全无定形的,但在 NMR 波谱中观察到明显不同的局部阴离子配位。将两个样品的光谱解卷积为三个峰,其中 PS43– 和 P2S74– 是目标 Li7P3S11 相的组成元素,P2S64– 是 Li4P2S6 杂质相的局部单位。(19) 图 1(c) 显示了对应于三个阴离子块的峰的峰的面积大小分数的比较。尽管玻璃相的性质通常包含各种局部结构,但样品 1 中 P2S 6 4– 的量少于样品 2 中的 P 2 S64– 量。 此外,样品 1 和 2 的 P2S74– 和 PS43– 的面积大小比分别为 1.70 和 1.24。样品 1 的面积尺寸比接近 2.00,这是结晶 Li7P3S11 中两种组分的理论化学计量比,表明样品 1 中的阴离子块与热处理前的 Li7P3S11 中的阴离子块相似。图 S2 显示了具有更大范围化学位移的数据,证实边带和主峰之间没有重叠。图 1.31(a) 样品 1 和 (b) 样品 2 的研磨粉末的 P MAS NMR 谱图和去卷积峰剖面。(c) 样品 1 和 2 的面积大小分数。原位同步加速器 X 射线衍射 (SXRD) 表明两种 Li2S-P2S5 玻璃之间的结晶过程明显不同(图 2)。样品 1 在大约 220 °C 时表现出单步结晶。 相反,样品 2 在大约 220 °C 时连续形成 Li3PS4,然后在大约 240 °C 下依次形成 Li7P3S11,因此经历了两步结晶过程。 对温度依赖性衍射图进行 Rietveld 精修,以量化不同温度下的 Li7P3S11、Li3PS4 和 Li4P2S6 馏分。样品 1 结晶成 Li7P3S11,没有明显的副相,而样品 2 即使在 300 °C 下也含有 11% 的 Li3PS4 和 2% 的 Li4P2S6,这会降低其离子传输性能。 (30−33) 衍射曲线未显示 Li7P3S11 相的峰存在显著差异(图 S3)。差热分析 (DTA) 证实了结晶过程的差异(图 S4)。虽然在样品 1 中观察到尖锐的放热信号,但在样品 2 中观察到具有两个不同峰的宽信号。即使对于不同批次的相同样品,这种趋势也是可重现的,这表明阴离子块在原始玻璃状态下的局部不均匀性,如 NMR 数据所示。图 2.在 N2 流速和相比下以 60 °C/min 加热的粉末的 SXRD 数据,来自 Rietveld 精炼的 (a) 样品 1 和 (b) 样品 2。由局部结构差异引起的明显不同的结晶过程导致所得结晶 Li7P3S11 相的性质发生变化。图 S5 显示了结晶后两个样品的 31P NMR 波谱。虽然由于样品 1 中出现交叉峰,热处理样品的光谱难以定量局部单位,但可以清楚地观察到定性差异。应该注意的是,这种与 P2S74– 和 PS43– 相关的交叉峰通常出现在结晶良好的 Li7P3S11 相中。(34) 为了进一步研究样品 1 和 2 的晶相差异,对样品 1 和 2 进行了对分布函数 (PDF) 分析。由于两个样品的数据仅在加热后才出现长程排序,因此两个样品基本相似。 然而,根据手工铣削工艺的不同,G(r) 在短程 ∼3.4 Å 的量程中出现了轻微的变化(图 S6)。加热后样品 1 和 2 的形态没有显示显着差异(图 S7)。总体而言,很明显,铣削前的预混合程序会严重影响最终结构。上述差异导致所得材料的离子传输特性存在显著差异。将在 280–300 °C 下结晶的粉末压制成颗粒,并通过温度依赖性阻抗谱测量其离子电导率,以分析样品中的锂离子传输。此外,根据 Arrhenius 关系确定锂离子传导的迁移势垒:图 3.样品 1 和 2 的温度和离子电导率的 Arrhenius 图。作为评估预混合影响的另一种模型系统,我们采用了一种新发现的卤化物基固体电解质 NaTaCl6 来进一步研究预混合的影响。据报道,NaTaCl6 的离子传输特性随其结晶度而显着变化。(27) 此外,已经揭示了机械化学合成的拉长研磨时间对离子传输性能的影响。(26) 在本节中,合成了四个样品,有或没有预混合,并进行了 16.5 或 100 小时的研磨。图 4 显示了在不同研磨时间(有或没有预混合)下获得的四个样品的室温离子电导率和活化能。未预混合的样品通过球磨合成;也就是说,前驱体与研磨介质一起直接放入球磨杯中。 同时,将预混样品与研钵和研杵充分混合后,再进行球磨。将研磨时间从 16.5 h 延长到 100 h 略微提高了离子电导率。然而,通过充分预混合前驱体,离子电导率得到了更显著的改善,而它们的 XRD 图谱没有显著差异。值得注意的是,研磨 100 小时不足以达到与预混合样品相同的离子传输水平。这些结果突出了预混合步骤的重要性。图 4.机械化学合成的 NaTaCl6 样品在不同条件下的离子电导率和活化能。每个样品合成 3 次,误差线是 3 个批次样品的标准差。Li7P3S11 和 NaTaCl6 合成强调了机械研磨前手工混合步骤对局部结构、结晶温度和离子电导率的关键影响。尽管需要进一步积累实验证据以精确阐明潜在机制,但目前的发现如下:在没有实施预混合步骤的情况下观察到局部单位分数的显着变化,表明局部不均匀性。这些局部成分变化会导致在随后的结晶过程中产生不同的阴离子嵌段,进而改变产品的结构和特性。 在 NaTaCl6 的情况下,延长球磨持续时间并不能消除局部成分变化,这从球磨时间从 16.5 小时增加到 100 小时时电导率的有限改善可以推断出来。同时,通过在机械化学合成之前引入简短的预混步骤,所得的离子传输得到了极大的改善。虽然延长研磨的影响可能因前驱体的化学和机械性能而异,但预混合程序有助于缩短合成时间以获得目标相。对研磨介质的粘附力差异被认为是成分不均匀的潜在原因。球磨将混合前驱体均质化,当它们被困在研磨介质之间或介质与杯内壁之间时。这个过程形成了具有各种成分的层状结构的粉末。随着层间距离的减小,机械混合促进了这种反应,从而减少了形成目标相所需的扩散长度。然而,当异质前驱体混合物被引入研磨杯中时,较软的材料往往会首先粘附在研磨介质上。因此,我们假设这形成了具有恒定扩散长度的厚层,导致明显的局部成分变化。起始材料和最终产物的机械性能对于反应的进行至关重要。 (35) 由于看似可重复的性质(离子电导率和循环性能)的显着变化是固态电池领域的巨大挑战之一,并引起了越来越多的关注,最近的报告展示了关键问题,(36,37) 我们对在看似稳健的球磨机械化学合成之前预混合程序的巨大影响的研究结果进一步强调了合成条件中细节的重要性。支持信息可在 https://pubs.acs.org/doi/10.1021/acsenergylett.4c02156 免费获取。Li7P3S11 和 NaTaCl6 的合成过程表、表征细节、Li7P3S11 的测量结果(XRD 图谱、NMR 光谱、DTA 曲线、PDF 和 SEM 图像) (PDF)细节很重要:揭示固体电解质机械化学合成中被忽视的参数 1 次浏览 0 分享 0 下载 大多数电子支持信息文件无需订阅 ACS Web 版本即可获得。此类文件可以按文章下载用于研究用途(如果有链接到相关文章的公共使用许可证,则该许可证可能允许其他用途)。可以通过 RightsLink 权限系统(http://pubs.acs.org/page/copyright/permissions.html)请求,从 ACS 获得用于其他用途的权限。A. Miura 感谢 Masae Sawamoto 女士为 SXRD 测量准备粉末毛细管。作者感谢东北大学的 Yuki Chiba 先生对固体 MAS NMR 测量的支持。S.O. 衷心感谢丰田理研通过 Rising Fellows 计划提供的财政支持。本研究得到了 JST PRESTO JPMJPR21Q8 、 JST Gtex JPMJGX23S5 和 JPMJGX23S2 的部分支持。本文引用了其他 37 种出版物。本文尚未被其他出版物引用。






























 京公网安备 11010802027423号
京公网安备 11010802027423号