当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery and Synthetic Applications of a NAD(P)H-Dependent Reductive Aminase from Rhodococcus erythropolis
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-12-16 , DOI: 10.1021/acscatal.4c04935
Ewald P. J. Jongkind, Jack Domenech, Arthur Govers, Marcel van den Broek, Jean-Marc Daran, Gideon Grogan, Caroline E. Paul
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-12-16 , DOI: 10.1021/acscatal.4c04935
Ewald P. J. Jongkind, Jack Domenech, Arthur Govers, Marcel van den Broek, Jean-Marc Daran, Gideon Grogan, Caroline E. Paul
|
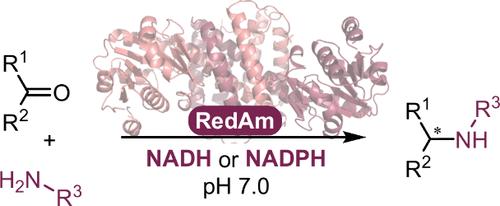
Reductive amination is one of the most synthetically direct routes to access chiral amines. Several Imine Reductases (IREDs) have been discovered to catalyze reductive amination (Reductive Aminases or RedAms), yet they are dependent on the expensive phosphorylated nicotinamide adenine dinucleotide cofactor NADPH and usually more active at basic pH. Here, we describe the discovery and synthetic potential of an IRED from Rhodococcus erythropolis (RytRedAm) that catalyzes reductive amination between a series of medium to large carbonyl and amine compounds with conversions of up to >99% and 99% enantiomeric excess at neutral pH. RytRedAm catalyzes the formation of a substituted γ-lactam and N-methyl-1-phenylethanamine with stereochemistry opposite to that of fungal RedAms, giving the (S)-enantiomer. This enzyme remarkably uses both NADPH and NADH cofactors with KM values of 15 and 247 μM and turnover numbers kcat of 3.6 and 9.0 s–1, respectively, for the reductive amination of hexanal with allylamine. The crystal structure obtained provides insights into the flexibility to also accept NADH, with residues R35 and I69 diverging from that of other IREDs/RedAms in the otherwise conserved Rossmann fold. RytRedAm thus represents a subfamily of enzymes that enable synthetic applications using NADH-dependent reductive amination to access complementary chiral amine products.
中文翻译:

红球菌 NAD(P)H 依赖性还原酶的发现和合成应用
还原胺化是获得手性胺的最合成直接途径之一。已经发现几种亚胺还原酶 (IRED) 可以催化还原胺化(还原胺化酶或 RedAms),但它们依赖于昂贵的磷酸化烟酰胺腺嘌呤二核苷酸辅因子 NADPH,通常在碱性 pH 值下更活跃。在这里,我们描述了来自红球菌(右RedAm)的 IRED 的发现和合成潜力,该 IRED 催化一系列中大型羰基和胺化合物之间的还原胺化反应,在中性 pH 值下转化率高达 >99% 和 99% 对映体过量。右RedAm 催化取代的 γ-内酰胺和 N-甲基-1-苯乙胺的形成,立体化学反应与真菌 RedAms 相反,得到 (S)-对映异构体。该酶显着使用 KM 值为 15 和 247 μM,周转数 kcat 分别为 3.6 和 9.0 s-1 的 NADPH 和 NADH 辅因子,用于己醛与烯丙胺的还原胺化。获得的晶体结构有助于了解接受 NADH 的灵活性,残基 R35 和 I69 与其他 IRED/RedAms 的残基不同,否则是保守的 Rossmann 折叠。右因此,RedAm 代表了一个酶亚家族,这些酶能够使用 NADH 依赖性还原胺化进行合成应用,以获得互补的手性胺产物。
更新日期:2024-12-16
中文翻译:

红球菌 NAD(P)H 依赖性还原酶的发现和合成应用
还原胺化是获得手性胺的最合成直接途径之一。已经发现几种亚胺还原酶 (IRED) 可以催化还原胺化(还原胺化酶或 RedAms),但它们依赖于昂贵的磷酸化烟酰胺腺嘌呤二核苷酸辅因子 NADPH,通常在碱性 pH 值下更活跃。在这里,我们描述了来自红球菌(右RedAm)的 IRED 的发现和合成潜力,该 IRED 催化一系列中大型羰基和胺化合物之间的还原胺化反应,在中性 pH 值下转化率高达 >99% 和 99% 对映体过量。右RedAm 催化取代的 γ-内酰胺和 N-甲基-1-苯乙胺的形成,立体化学反应与真菌 RedAms 相反,得到 (S)-对映异构体。该酶显着使用 KM 值为 15 和 247 μM,周转数 kcat 分别为 3.6 和 9.0 s-1 的 NADPH 和 NADH 辅因子,用于己醛与烯丙胺的还原胺化。获得的晶体结构有助于了解接受 NADH 的灵活性,残基 R35 和 I69 与其他 IRED/RedAms 的残基不同,否则是保守的 Rossmann 折叠。右因此,RedAm 代表了一个酶亚家族,这些酶能够使用 NADH 依赖性还原胺化进行合成应用,以获得互补的手性胺产物。































 京公网安备 11010802027423号
京公网安备 11010802027423号