当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Construction of Chiral C2-Quaternary Indolines via Palladium-Catalyzed Decarboxylative Asymmetric Amination
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-12-16 , DOI: 10.1021/acscatal.4c05763 Mingjun Lv, Xinhui Yu, Jitian Liu, Xiaoxun Li
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-12-16 , DOI: 10.1021/acscatal.4c05763 Mingjun Lv, Xinhui Yu, Jitian Liu, Xiaoxun Li
|
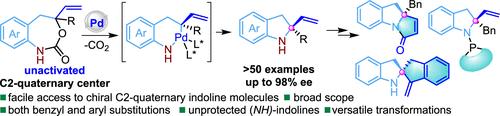
The catalytic asymmetric synthesis of functionalized C2-quaternary indoline scaffolds has garnered significant attention in organic synthesis and drug discovery due to the inherent challenges and potential applications. Herein, we present a facile approach utilizing a Pd-catalyzed intramolecular decarboxylative asymmetric amination of vinyl benzoxazepinones, leading to the efficient construction of challenging chiral 2-vinyl-2-aryl/alkyl indoline frameworks in good yields with high enantioselectivities (>50 examples, up to 83% yield and 97% ee). Furthermore, these chiral indolines can be readily scaled up and further modified to access complex polycyclic indoline structures. We also synthesized several indoline-based ligands that exhibit promising efficiency as chiral catalysts in asymmetric reactions. Computational studies provided insight into the inner-sphere asymmetric amination mechanism.
中文翻译:

通过钯催化的脱羧不对称胺化构建手性 C2-季吲哚啉
由于固有的挑战和潜在应用,功能化 C2-季吲哚啉支架的催化不对称合成在有机合成和药物发现中引起了广泛关注。在此,我们提出了一种利用乙烯基苯并噁啉酮的分子内脱羧不对称胺化的简单方法,从而以高对映选择性的良好产量高效构建具有挑战性的手性 2-乙烯基-2-芳基/烷基吲哚啉框架(>50 示例,高达 83% 的产率和 97% ee)。此外,这些手性吲哚啉可以很容易地放大和进一步修饰以获得复杂的多环吲哚啉结构。我们还合成了几种基于吲哚啉的配体,这些配体在不对称反应中作为手性催化剂表现出有希望的效率。计算研究提供了对球内不对称胺化机制的见解。
更新日期:2024-12-17
中文翻译:

通过钯催化的脱羧不对称胺化构建手性 C2-季吲哚啉
由于固有的挑战和潜在应用,功能化 C2-季吲哚啉支架的催化不对称合成在有机合成和药物发现中引起了广泛关注。在此,我们提出了一种利用乙烯基苯并噁啉酮的分子内脱羧不对称胺化的简单方法,从而以高对映选择性的良好产量高效构建具有挑战性的手性 2-乙烯基-2-芳基/烷基吲哚啉框架(>50 示例,高达 83% 的产率和 97% ee)。此外,这些手性吲哚啉可以很容易地放大和进一步修饰以获得复杂的多环吲哚啉结构。我们还合成了几种基于吲哚啉的配体,这些配体在不对称反应中作为手性催化剂表现出有希望的效率。计算研究提供了对球内不对称胺化机制的见解。






























 京公网安备 11010802027423号
京公网安备 11010802027423号