当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Self-assembly of cationic surfactant in choline chloride-based deep eutectic solvents: structural solvation and dynamics
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2024-12-17 , DOI: 10.1039/d4cp02739f Yagnik Vora, Omish Sethi, Sanjay N. Bariya, Santosh L. Gawali, Saurabh S. Soni, Tejwant Singh Kang, Puthusserickal A. Hassan, Ketan Kuperkar
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2024-12-17 , DOI: 10.1039/d4cp02739f Yagnik Vora, Omish Sethi, Sanjay N. Bariya, Santosh L. Gawali, Saurabh S. Soni, Tejwant Singh Kang, Puthusserickal A. Hassan, Ketan Kuperkar
|
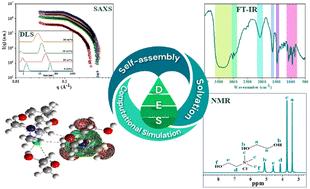
Deep eutectic solvents (DESs) have gained popularity in various applications due to their improved environmental sustainability and biodegradability. For the present study, several polyhydric alcohols, including ethylene glycol (EG), diethylene glycol (DEG), triethylene glycol (TEG), and glycerol (Gly), have been used as hydrogen bond donors (HBDs) and choline chloride (ChCl) as a hydrogen bond acceptor (HBA) in a fixed molar ratio to form a homogenous and stable DES. Controlled water mixing into such neat DESs has always been thought to be a quick and efficient method to tune the chemical and thermodynamic properties of DESs. The structural solvation and dynamics of the prepared DESs with the inclusion of water vary from low to high water concentrations that have been examined employing Fourier transform infrared (FT-IR), and proton nuclear magnetic resonance (1H-NMR) spectroscopy. Herein, the micellization behavior of a cationic surfactant, dodecyltrimethylammonium bromide (DTAB), in neat DESs and DES–water mixtures has been demonstrated by using tensiometry, dynamic light scattering (DLS), and small-angle X-ray scattering (SAXS) techniques. Furthermore, it has been observed that DESs exhibit an ample thermodynamic driving force for creating micelles, since they contain an H-bonded nanostructure. However, such self-assembly appears to be very much dependent on DESs, the amount of water, and the surfactant used. A computational simulation approach using a semiempirical method is put forth employing the Gaussian 09 W calculation window in the Gauss View 5.0.9 software package. In addition, this study includes the determination of several optimized descriptors that intend to offer an in-depth examination of the surfactant–DES interactions.
中文翻译:

阳离子表面活性剂在氯化胆碱基深共熔溶剂中的自组装:结构溶剂化和动力学
深共熔溶剂 (DES) 因其改进的环境可持续性和生物降解性而在各种应用中广受欢迎。在本研究中,几种多元醇,包括乙二醇 (EG)、二甘醇 (DEG)、三甘醇 (TEG) 和甘油 (Gly),已用作氢键供体 (HBD),氯化胆碱 (ChCl) 作为氢键受体 (HBA),以固定摩尔比形成均匀稳定的 DES。将受控的水混合到这种纯 DES 中一直被认为是调节 DES 化学和热力学特性的快速有效的方法。所制备的含有水的 DES 的结构溶剂化和动力学从低水浓度到高水浓度不等,已使用傅里叶变换红外 (FT-IR) 和质子核磁共振 (1 H-NMR) 光谱进行了检查。在此,阳离子表面活性剂十二烷基三甲基溴化铵 (DTAB) 在纯 DES 和 DES-水混合物中的胶束化行为已通过使用张力测定法、动态光散射 (DLS) 和小角 X 射线散射 (SAXS) 技术得到证明。此外,据观察,DES 表现出足够的热力学驱动力来产生胶束,因为它们包含 H 键合纳米结构。然而,这种自组装似乎在很大程度上取决于 DES、水量和使用的表面活性剂。提出了一种采用半经验方法的计算仿真方法,采用 Gauss View 5.0.9 软件包中的 Gaussian 09 W 计算窗口。此外,本研究还包括确定几个优化的描述符,这些描述符旨在深入检查表面活性剂-DES 相互作用。
更新日期:2024-12-17
中文翻译:

阳离子表面活性剂在氯化胆碱基深共熔溶剂中的自组装:结构溶剂化和动力学
深共熔溶剂 (DES) 因其改进的环境可持续性和生物降解性而在各种应用中广受欢迎。在本研究中,几种多元醇,包括乙二醇 (EG)、二甘醇 (DEG)、三甘醇 (TEG) 和甘油 (Gly),已用作氢键供体 (HBD),氯化胆碱 (ChCl) 作为氢键受体 (HBA),以固定摩尔比形成均匀稳定的 DES。将受控的水混合到这种纯 DES 中一直被认为是调节 DES 化学和热力学特性的快速有效的方法。所制备的含有水的 DES 的结构溶剂化和动力学从低水浓度到高水浓度不等,已使用傅里叶变换红外 (FT-IR) 和质子核磁共振 (1 H-NMR) 光谱进行了检查。在此,阳离子表面活性剂十二烷基三甲基溴化铵 (DTAB) 在纯 DES 和 DES-水混合物中的胶束化行为已通过使用张力测定法、动态光散射 (DLS) 和小角 X 射线散射 (SAXS) 技术得到证明。此外,据观察,DES 表现出足够的热力学驱动力来产生胶束,因为它们包含 H 键合纳米结构。然而,这种自组装似乎在很大程度上取决于 DES、水量和使用的表面活性剂。提出了一种采用半经验方法的计算仿真方法,采用 Gauss View 5.0.9 软件包中的 Gaussian 09 W 计算窗口。此外,本研究还包括确定几个优化的描述符,这些描述符旨在深入检查表面活性剂-DES 相互作用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号