当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Investigation of charge transfer models on the evolution of phases in lithium iron phosphate batteries using phase-field simulations
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2024-12-17 , DOI: 10.1039/d4ta06444e Souzan Hammadi, Peter Broqvist, Daniel Brandell, Nana Ofori-Opoku
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2024-12-17 , DOI: 10.1039/d4ta06444e Souzan Hammadi, Peter Broqvist, Daniel Brandell, Nana Ofori-Opoku
|
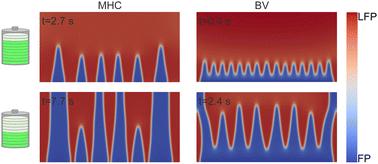
Charge transfer is essential for all electrochemical processes, such as in batteries where it is facilitated through the incorporation of ion–electron pairs into solid crystals. The low solubility of lithium (Li) in some of these host lattices cause phase changes, which for example happens in FePO4. This results in the growth of interfacial patterns at the mesoscale between a Li-poor and Li-rich phase, FePO4 and LiFePO4 respectively. Conventionally, the effect of charge transfer on the evolution of these phases is usually modelled using the Butler–Volmer equation. However, the exponentially increasing current–overpotential relation in this formalism becomes problematic for battery systems operating under high currents. In this study, we implement a phase-field model to investigate two electrochemical reaction models: the Butler–Volmer and the Marcus–Hush–Chidsey formulation. We assess their effect on the spatial and temporal evolution of the FePO4 and LiFePO4 phases. Both reaction models demonstrate similar microstructural patterns in equilibrium. Nevertheless, a significant increase in current density is caused by using the Butler–Volmer expression, leading to an accelerated reaction rate at high overpotentials and an exaggerated delithiation. Furthermore, we show that including anisotropic elastic strain fields in the phase-field model accelerates the delithiation process, reaching the bulk mass transport limitation faster. These elastic effects, when included in the overpotential, can cause the current density to exceed its limits, a problem inherently mitigated by the Marcus–Hush–Chidsey model.
中文翻译:

使用相场模拟研究磷酸铁锂电池相演变的电荷转移模型
电荷转移对于所有电化学过程都是必不可少的,例如在电池中,电荷转移是通过将离子-电子对掺入固体晶体来促进的。锂 (Li) 在其中一些主晶格中的低溶解度会导致相变,例如发生在 FePO4 中。这导致在贫锂相和富锂相之间界面图案的生长,分别为 FePO4 和 LiFePO4。传统上,电荷转移对这些相演变的影响通常使用 Butler-Volmer 方程进行建模。然而,这种形式中呈指数级增长的电流-过电位关系对于在大电流下运行的电池系统来说变得成问题。在这项研究中,我们实现了一个相场模型来研究两种电化学反应模型:Butler-Volmer 和 Marcus-Hush-Chidsey 公式。我们评估了它们对 FePO4 和 LiFePO4 期的空间和时间演变的影响。两种反应模型在平衡时都表现出相似的微观结构模式。然而,使用 Butler-Volmer 表达式会导致电流密度的显着增加,从而导致在高过电位下反应速率加快和夸大的脱锂反应。此外,我们表明,在相场模型中包含各向异性弹性应变场可以加速脱锂过程,更快地达到体质传递极限。当这些弹性效应包含在过电位中时,会导致电流密度超过其极限,Marcus-Hush-Chidsey 模型本身就缓解了这个问题。
更新日期:2024-12-17
中文翻译:

使用相场模拟研究磷酸铁锂电池相演变的电荷转移模型
电荷转移对于所有电化学过程都是必不可少的,例如在电池中,电荷转移是通过将离子-电子对掺入固体晶体来促进的。锂 (Li) 在其中一些主晶格中的低溶解度会导致相变,例如发生在 FePO4 中。这导致在贫锂相和富锂相之间界面图案的生长,分别为 FePO4 和 LiFePO4。传统上,电荷转移对这些相演变的影响通常使用 Butler-Volmer 方程进行建模。然而,这种形式中呈指数级增长的电流-过电位关系对于在大电流下运行的电池系统来说变得成问题。在这项研究中,我们实现了一个相场模型来研究两种电化学反应模型:Butler-Volmer 和 Marcus-Hush-Chidsey 公式。我们评估了它们对 FePO4 和 LiFePO4 期的空间和时间演变的影响。两种反应模型在平衡时都表现出相似的微观结构模式。然而,使用 Butler-Volmer 表达式会导致电流密度的显着增加,从而导致在高过电位下反应速率加快和夸大的脱锂反应。此外,我们表明,在相场模型中包含各向异性弹性应变场可以加速脱锂过程,更快地达到体质传递极限。当这些弹性效应包含在过电位中时,会导致电流密度超过其极限,Marcus-Hush-Chidsey 模型本身就缓解了这个问题。






























 京公网安备 11010802027423号
京公网安备 11010802027423号