当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electronic Structure Modulated by B-Doped Cu Promotes Electrocatalytic Nitrate Reduction for Ammonia Production
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-12-16 , DOI: 10.1021/acscatal.4c05954 Jiajia Wang, Zhuodong Ou, Chengbo Dong, Mengying Su, Amjad Ali, Artem V. Kuklin, Hans Ågren, Glib V. Baryshnikov, Yang Liu, Xue Zhao, Haibo Zhang
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-12-16 , DOI: 10.1021/acscatal.4c05954 Jiajia Wang, Zhuodong Ou, Chengbo Dong, Mengying Su, Amjad Ali, Artem V. Kuklin, Hans Ågren, Glib V. Baryshnikov, Yang Liu, Xue Zhao, Haibo Zhang
|
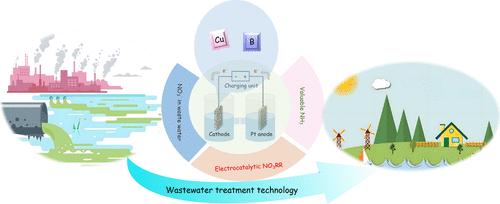
Electrocatalytic nitrate reduction for ammonia (eNIRR) is an ammonia production process that simultaneously removes nitrate contaminants from water. However, the lack of activity of cathode catalysts used as eNIRR catalysts is the main limiting factor for its development. Motivated by this fact, born-doped copper (BDCu) was obtained by using ZnO, which was easily removed at high temperature, as a dispersant, combined with weakly reducing boron clusters (closo-[B12H12]2–) as a reducing agent and B source during high-temperature pyrolysis. Impressively, BDCu demonstrated a Faradaic efficiency of 96.58% and a yield rate of 25741.51 μg h–1 mgcat–1 toward ammonia production at −1.8 V (vs saturated calomel electrode). The ammonia yield rate of BDCu was twice as high as in the case of undoped B. Evolutionary behavior of NO3– to NH3 conversion detected by in situ Fourier-transform infrared (in situ FT-IR) and electrochemical in situ mass spectrometry (in situ DEMS). Experimental and density functional theory (DFT) calculations explained that the activation of water was enhanced by B-doped Cu, and the adsorption of proton *H was weakened, which made it easy for *H to migrate away from the catalyst to NO3– as a proton required for NO3– reduction. In addition, the electron-deficient of B provides conditions for electron transfer between B and Cu. The electron transfer from Cu to B in BDCu led to a decrease in the center of the d-band of Cu, which modulated the electronic properties of Cu and altered the behavior of the NO3– to NH3 transition on the Cu surface. Compared with Cu undoped B as well as unreduced CuO, BDCu lowered the energy barrier of the rate-determining step (*NO → *N), allowing for a smoother conversion of NO3– to NH3. This study provides a strategy to change the electronic structure of transition metals by B-modification and thus improve the performance of ammonia synthesis.
中文翻译:

由 B 掺杂 Cu 调制的电子结构促进电催化硝酸盐还原以生产氨
电催化硝酸盐还原氨 (eNIRR) 是一种氨生产工艺,可同时去除水中的硝酸盐污染物。然而,用作 eNIRR 催化剂的阴极催化剂缺乏活性是其发展的主要限制因素。受此启发,以高温下易去除的 ZnO 为分散剂,结合弱还原硼簇 (closo-[B12H12]2–) 作为还原剂和高温热解过程中的 B 源,获得了原生掺杂铜 (BDCu)。令人印象深刻的是,BDCu 在 -1.8 V 下生产氨的法拉第效率为 96.58%,产率为 25741.51 μg h–1 mgcat–1(与饱和甘汞电极相比)。BDCu 的氨产率是未掺杂 B 的两倍。通过原位傅里叶变换红外(原位 FT-IR)和电化学原位质谱法(原位 DEMS)检测到 NO3– 到 NH3 转化的进化行为。实验和密度泛函理论 (DFT) 计算表明,B 掺杂的 Cu 增强了水的活化,并且减弱了质子 *H 的吸附,这使得 *H 很容易从催化剂迁移到 NO3– 作为 NO3– 还原所需的质子。此外,B 的缺电子为 B 和 Cu 之间的电子转移提供了条件。BDCu 中从 Cu 到 B 的电子转移导致 Cu 的 d 带中心减小,这调节了 Cu 的电子性质并改变了 Cu 表面 NO3– 到 NH3 转变的行为。 与未掺杂 Cu B 和未还原的 CuO 相比,BDCu 降低了速率确定步骤的能量势垒 (*NO → *N),从而允许 NO3– 更平滑地转化为 NH3。本研究提供了一种通过 B 修饰改变过渡金属电子结构的策略,从而提高氨合成的性能。
更新日期:2024-12-16
中文翻译:

由 B 掺杂 Cu 调制的电子结构促进电催化硝酸盐还原以生产氨
电催化硝酸盐还原氨 (eNIRR) 是一种氨生产工艺,可同时去除水中的硝酸盐污染物。然而,用作 eNIRR 催化剂的阴极催化剂缺乏活性是其发展的主要限制因素。受此启发,以高温下易去除的 ZnO 为分散剂,结合弱还原硼簇 (closo-[B12H12]2–) 作为还原剂和高温热解过程中的 B 源,获得了原生掺杂铜 (BDCu)。令人印象深刻的是,BDCu 在 -1.8 V 下生产氨的法拉第效率为 96.58%,产率为 25741.51 μg h–1 mgcat–1(与饱和甘汞电极相比)。BDCu 的氨产率是未掺杂 B 的两倍。通过原位傅里叶变换红外(原位 FT-IR)和电化学原位质谱法(原位 DEMS)检测到 NO3– 到 NH3 转化的进化行为。实验和密度泛函理论 (DFT) 计算表明,B 掺杂的 Cu 增强了水的活化,并且减弱了质子 *H 的吸附,这使得 *H 很容易从催化剂迁移到 NO3– 作为 NO3– 还原所需的质子。此外,B 的缺电子为 B 和 Cu 之间的电子转移提供了条件。BDCu 中从 Cu 到 B 的电子转移导致 Cu 的 d 带中心减小,这调节了 Cu 的电子性质并改变了 Cu 表面 NO3– 到 NH3 转变的行为。 与未掺杂 Cu B 和未还原的 CuO 相比,BDCu 降低了速率确定步骤的能量势垒 (*NO → *N),从而允许 NO3– 更平滑地转化为 NH3。本研究提供了一种通过 B 修饰改变过渡金属电子结构的策略,从而提高氨合成的性能。






























 京公网安备 11010802027423号
京公网安备 11010802027423号