当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Compatibility of Halide Electrolytes in Solid-State Li–S Battery Cathodes
Chemistry of Materials ( IF 7.2 ) Pub Date : 2024-12-16 , DOI: 10.1021/acs.chemmater.4c02159 Shoma Yanagihara, Jan Huebner, Zheng Huang, Atsushi Inoishi, Hirofumi Akamatsu, Katsuro Hayashi, Saneyuki Ohno
Chemistry of Materials ( IF 7.2 ) Pub Date : 2024-12-16 , DOI: 10.1021/acs.chemmater.4c02159 Shoma Yanagihara, Jan Huebner, Zheng Huang, Atsushi Inoishi, Hirofumi Akamatsu, Katsuro Hayashi, Saneyuki Ohno
|
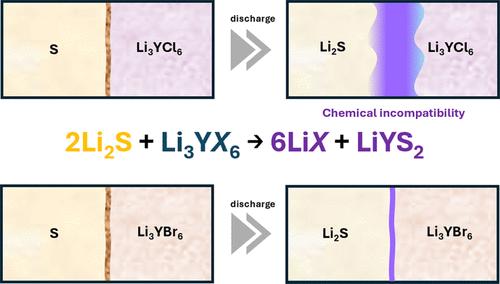
The utilization of earth-abundant and high-capacity sulfur in solid-state batteries presents a promising strategy to circumvent the use of rare transition metals and enhance achievable specific energy. However, numerous challenges remain. The transport limitation within the cathode composite, particularly with sulfide electrolytes during charging, has been identified as a major degradation mechanism in solid-state Li–S batteries. This degradation is linked to electrolyte oxidation and a concomitant reduction in the effective ionic conductivity of the cathode composite. Inspired by the sufficiently high oxidation stability of halide-based electrolytes, we investigated their compatibility with solid-state Li–S batteries in this work. The electrochemical stability of halides in contact with conductive additives, the stability window of fast ion transport in the composite electrodes, and chemical compatibility with sulfur-active materials (e.g., S and Li2S), in addition to the cyclability of the halide-based composite electrodes, are explored. Three halides were employed as model electrolytes: Li3InCl6, Li3YCl6, and Li3YBr6. Despite its high oxidation stability, Li3InCl6 exhibited rapid degradation due to electrolyte reduction. The composite with Li3YCl6 lost its capacity because of chemical incompatibility, especially with Li2S, resulting in the formation of LiYS2 at the interface. In contrast, Li3YBr6 demonstrated superior performance, maintaining a capacity of 1100 mAh gS–1 for 20 cycles (normalized to the sulfur content in the cathode material). This study elucidates the degradation mechanisms of halide-based solid-state Li–S batteries and proposes potential design strategies to mitigate chemical incompatibility issues.
中文翻译:

卤化物电解质在固态 Li-S 电池阴极中的相容性
在固态电池中利用地球丰富的高容量硫提供了一种很有前途的策略,可以避免使用稀有过渡金属并提高可实现的比能。然而,仍然存在许多挑战。阴极复合材料内的传输限制,特别是充电过程中硫化物电解质的传输限制,已被确定为固态 Li-S 电池的主要降解机制。这种降解与电解质氧化和随之而来的阴极复合材料有效离子电导率的降低有关。受卤化物基电解质足够高的氧化稳定性的启发,我们在这项工作中研究了它们与固态 Li-S 电池的相容性。除了卤化物基复合电极的可循环性外,还探讨了卤化物与导电添加剂接触的电化学稳定性、复合电极中快速离子传输的稳定性窗口以及与硫活性材料(例如 S 和 Li 2 S)的化学相容性。采用三种卤化物作为模型电解质:Li 3 InCl 6 、Li 3 YCl 6 和 Li 3 YBr 6 。尽管 Li 3 InCl 具有很高的氧化稳定性,但由于电解质还原而 6 表现出快速降解。与 Li 3 YCl 6 的复合材料由于化学不相容而失去了容量,尤其是与 Li 2 S 的不相容性,导致在界面处形成 LiYS 2 。相比之下,Li 3 YBr 6 表现出卓越的性能,在 20 次循环中保持 1100 mAh g S –1 的容量(归一化为正极材料中的硫含量)。 本研究阐明了卤化物基固态 Li-S 电池的降解机制,并提出了缓解化学不相容性问题的潜在设计策略。
更新日期:2024-12-16
中文翻译:

卤化物电解质在固态 Li-S 电池阴极中的相容性
在固态电池中利用地球丰富的高容量硫提供了一种很有前途的策略,可以避免使用稀有过渡金属并提高可实现的比能。然而,仍然存在许多挑战。阴极复合材料内的传输限制,特别是充电过程中硫化物电解质的传输限制,已被确定为固态 Li-S 电池的主要降解机制。这种降解与电解质氧化和随之而来的阴极复合材料有效离子电导率的降低有关。受卤化物基电解质足够高的氧化稳定性的启发,我们在这项工作中研究了它们与固态 Li-S 电池的相容性。除了卤化物基复合电极的可循环性外,还探讨了卤化物与导电添加剂接触的电化学稳定性、复合电极中快速离子传输的稳定性窗口以及与硫活性材料(例如 S 和 Li 2 S)的化学相容性。采用三种卤化物作为模型电解质:Li 3 InCl 6 、Li 3 YCl 6 和 Li 3 YBr 6 。尽管 Li 3 InCl 具有很高的氧化稳定性,但由于电解质还原而 6 表现出快速降解。与 Li 3 YCl 6 的复合材料由于化学不相容而失去了容量,尤其是与 Li 2 S 的不相容性,导致在界面处形成 LiYS 2 。相比之下,Li 3 YBr 6 表现出卓越的性能,在 20 次循环中保持 1100 mAh g S –1 的容量(归一化为正极材料中的硫含量)。 本研究阐明了卤化物基固态 Li-S 电池的降解机制,并提出了缓解化学不相容性问题的潜在设计策略。






























 京公网安备 11010802027423号
京公网安备 11010802027423号