当前位置:
X-MOL 学术
›
Adv. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Engineered Sodium Metal Anodes: Tackling Sulfur‐Derivative Challenges for Advanced Sodium–Sulfur Batteries
Advanced Energy Materials ( IF 24.4 ) Pub Date : 2024-12-16 , DOI: 10.1002/aenm.202404901 Qing Zhao, Tiehan Mei, Yi Li, Xitao Lin, Yubin Niu, Jian Jiang, Maowen Xu, Yuruo Qi
Advanced Energy Materials ( IF 24.4 ) Pub Date : 2024-12-16 , DOI: 10.1002/aenm.202404901 Qing Zhao, Tiehan Mei, Yi Li, Xitao Lin, Yubin Niu, Jian Jiang, Maowen Xu, Yuruo Qi
|
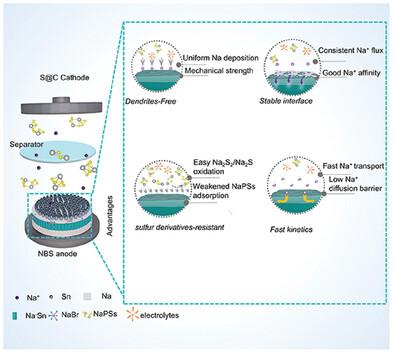
The development of room temperature sodium–sulfur (RT Na─S) batteries has been significantly constrained by the dissolution/shuttle of sulfur‐derivatives and the instability of sodium anode. This study presents an engineered sodium metal anode (NBS), featuring sodium bromide (NaBr) along with sodiophilic components like tin metal (Sn) and sodium‐tin (Na─Sn) alloy. This configuration exhibits high plating/stripping reversibility with minimal nucleation/growth barriers in an ester‐based electrolyte, allowing stable cycling of symmetric cells at 2 mA cm⁻2 /2 mA h cm⁻2 for over 2000 h at a low overpotential of 30 mV. Importantly, the weak adsorption and reduced electron transfer towards sulfur‐derivatives, along with the facile dissociation of Na2 S2 /Na2 S, effectively minimize the accumulation of sulfur‐derivatives, thereby improving the interfacial stability of the NBS electrode in sulfur‐derivatives‐involved conditions. As a result, the NBS anode endows the Na─S full cells paired with either a Co‐NMCN@S or SPAN cathode superior electrochemical performance, with the SPAN//NBS system delivering an outstanding reversible capacity of 1639.5 mA h g⁻¹ and a low degradation rate of 0.06% per cycle at 0.5C. This study elucidates the complex deposition/dissolution kinetics and interface chemistry associated with sulfur species, providing valuable insights for enhancing sodium anodes in practical RT Na─S systems.
中文翻译:

工程钠金属负极:应对先进钠硫电池的硫衍生物挑战
室温钠-硫 (RT Na─S) 电池的发展受到硫衍生物的溶解/穿梭和钠负极不稳定性的显著限制。本研究提出了一种工程金属钠阳极 (NBS),其特点是溴化钠 (NaBr) 以及锡金属 (Sn) 和钠锡 (Na─Sn) 合金等嗜钠成分。这种配置在酯基电解质中表现出高电镀/剥离可逆性,成核/生长障碍最小,允许在 30 mV 的低过电位下,对称电池在 2 mA cm⁻2/2 mA h cm⁻2 下稳定循环超过 2000 小时。重要的是,Na2S2/Na2S 的弱吸附和减少的电子向硫衍生物的转移,以及 Na2S2/Na2S 的轻松解离,有效地最大限度地减少了硫衍生物的积累,从而提高了 NBS 电极在硫衍生物参与条件下的界面稳定性。因此,NBS 负极赋予 Na─S 全电池与 Co-NMCN@S 或 SPAN 阴极配对的卓越电化学性能,SPAN//NBS 系统在 0.5C 下提供 1639.5 mA h g⁻¹ 的出色可逆容量和每循环 0.06% 的低降解率。本研究阐明了与硫种类相关的复杂沉积/溶解动力学和界面化学,为在实际 RT Na─S 系统中增强钠阳极提供了有价值的见解。
更新日期:2024-12-16
中文翻译:

工程钠金属负极:应对先进钠硫电池的硫衍生物挑战
室温钠-硫 (RT Na─S) 电池的发展受到硫衍生物的溶解/穿梭和钠负极不稳定性的显著限制。本研究提出了一种工程金属钠阳极 (NBS),其特点是溴化钠 (NaBr) 以及锡金属 (Sn) 和钠锡 (Na─Sn) 合金等嗜钠成分。这种配置在酯基电解质中表现出高电镀/剥离可逆性,成核/生长障碍最小,允许在 30 mV 的低过电位下,对称电池在 2 mA cm⁻2/2 mA h cm⁻2 下稳定循环超过 2000 小时。重要的是,Na2S2/Na2S 的弱吸附和减少的电子向硫衍生物的转移,以及 Na2S2/Na2S 的轻松解离,有效地最大限度地减少了硫衍生物的积累,从而提高了 NBS 电极在硫衍生物参与条件下的界面稳定性。因此,NBS 负极赋予 Na─S 全电池与 Co-NMCN@S 或 SPAN 阴极配对的卓越电化学性能,SPAN//NBS 系统在 0.5C 下提供 1639.5 mA h g⁻¹ 的出色可逆容量和每循环 0.06% 的低降解率。本研究阐明了与硫种类相关的复杂沉积/溶解动力学和界面化学,为在实际 RT Na─S 系统中增强钠阳极提供了有价值的见解。






























 京公网安备 11010802027423号
京公网安备 11010802027423号