当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Fragment Discovery by X-Ray Crystallographic Screening Targeting the CTP Binding Site of Pseudomonas Aeruginosa IspD
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2024-12-15 , DOI: 10.1002/anie.202414615 Daan Willocx, Lucia D'Auria, Danica Walsh, Hugo Scherer, Alaa Alhayek, Mostafa M. Hamed, Franck Borel, Eleonora Diamanti, Anna K. H. Hirsch
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2024-12-15 , DOI: 10.1002/anie.202414615 Daan Willocx, Lucia D'Auria, Danica Walsh, Hugo Scherer, Alaa Alhayek, Mostafa M. Hamed, Franck Borel, Eleonora Diamanti, Anna K. H. Hirsch
|
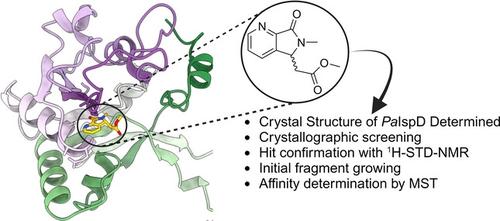
During a crystallographic screening with IspD from Pseudomonas aeruginosa, we identified a fragment residing inside the active site. We confirmed its interactions with the protein by 1H saturation transfer difference NMR spectroscopy. Initial fragment growing yielded derivatives with improved affinity towards IspD from Pseudomonas aeruginosa.
中文翻译:

通过靶向铜绿假单胞菌 IspD 的 CTP 结合位点的 X 射线晶体学筛选发现片段
在用铜绿假单胞菌的 IspD 进行晶体学筛选时,我们鉴定了位于活性位点内的片段。我们通过 1H 饱和转移差分 NMR 波谱证实了它与蛋白质的相互作用。初始片段生长产生的衍生物对铜绿假单胞菌的 IspD 具有更高的亲和力。
更新日期:2024-12-15
中文翻译:

通过靶向铜绿假单胞菌 IspD 的 CTP 结合位点的 X 射线晶体学筛选发现片段
在用铜绿假单胞菌的 IspD 进行晶体学筛选时,我们鉴定了位于活性位点内的片段。我们通过 1H 饱和转移差分 NMR 波谱证实了它与蛋白质的相互作用。初始片段生长产生的衍生物对铜绿假单胞菌的 IspD 具有更高的亲和力。

































 京公网安备 11010802027423号
京公网安备 11010802027423号