当前位置:
X-MOL 学术
›
Water Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Differentiating reactive chlorine species for micropollutant abatement in chloride containing water by electrochemical oxidation process
Water Research ( IF 11.4 ) Pub Date : 2024-12-15 , DOI: 10.1016/j.watres.2024.122984 Weikang Lai, Xin Yang, Zhechao Hua, Anna Wang, Dequan He, Zhipeng Wei, Ming Yang, Jingyun Fang
Water Research ( IF 11.4 ) Pub Date : 2024-12-15 , DOI: 10.1016/j.watres.2024.122984 Weikang Lai, Xin Yang, Zhechao Hua, Anna Wang, Dequan He, Zhipeng Wei, Ming Yang, Jingyun Fang
|
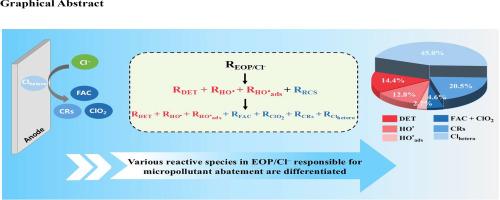
Electrochemical oxidation process (EOP) is promising for micropollutant degradation in water treatment, where chloride ions (Cl– ) are inevitable in aqueous systems, leading to the EOP/Cl– system. The oxidation of Cl– at anodes generates reactive chlorine species (RCS), including heterogeneous chlorine species (Clhetero ), homogeneous free available chlorine (FAC), chlorine dioxide (ClO2 ), and chlorine radicals (CRs). This study developed a method to differentiate various RCS responsible for the removal of carbamazepine in EOP/Cl– using the RuO2 /IrO2 -Ti anode. Compared to EOP, the formation of RCS significantly enhanced the degradation of carbamazepine in EOP/Cl– , primarily through heterogeneous Clhetero , homogeneous molecular chlorine (Cl2 ), and CRs. The relative contribution of specific RCS to carbamazepine degradation significantly varied at different pHs, Cl– concentrations, and current densities. As pH increased from 5.3 to 10.0 with 10 mM Cl– , the relative contributions of Clhetero and CRs decreased, while Clhetero dominated carbamazepine degradation at pH 7.0 and 10.0. Cl2 was the dominant species for carbamazepine degradation at pH 5.3, while its role significantly decreased at higher pHs. The increase of Cl– concentrations enhanced the relative contributions of Clhetero , Cl2 , and CRs at pH 5.3 and 18 mA/cm2 . The rise of current density from 18 to 39 mA/cm2 significantly promoted the relative contributions of Clhetero and CRs at pH 7.0 and 10 mM Cl– . This study elucidated the specific roles of reactive species for micropollutant degradation in EOP/Cl– , highlighting the significance of heterogeneous Clhetero and homogeneous CRs and Cl2 .
中文翻译:

通过电化学氧化工艺区分活性氯种类以去除含氯水中微污染物
电化学氧化过程 (EOP) 有望在水处理中降解微污染物,其中氯离子 (Cl–) 在水性系统中是不可避免的,从而导致 EOP/Cl– 系统。Cl 的氧化– 在阳极产生活性氯物质 (RCS),包括异质氯物质 (Clhetero)、均相游离有效氯 (FAC)、二氧化氯 (ClO2) 和氯自由基 (CR)。本研究开发了一种区分负责去除 EOP/Cl 中卡马西平的各种 RCS 的方法– 使用 RuO2/IrO2-Ti 阳极。与 EOP 相比,RCS 的形成显着增强了卡马西平在 EOP/Cl 中的降解–主要通过异质 Clhetero、均相分子氯 (Cl2) 和 CRs。特异性 RCS 对卡马西平降解的相对贡献在不同 pH 值、Cl– 浓度和电流密度下显著变化。当 pH 值从 5.3 增加到 10.0 时,10 mM Cl–,Clhetero 和 CRs 的相对贡献降低,而 Clhetero 在 pH 值为 7.0 和 10.0 时主导卡马西平降解。Cl2 在 pH 值为 5.3 时是卡马西平降解的优势物质,而在较高 pH 值下其作用显著降低。Cl– 浓度的增加增强了 Clhetero、Cl2 和 CRs 在 pH 5.3 和 18 mA/cm2 下的相对贡献。电流密度从 18 mA/cm2 增加到 39 mA/cm2 显着促进了 Clhetero 和 CR 在 pH 7.0 和 10 mM Cl 下的相对贡献–。本研究阐明了反应性物质对 EOP/Cl 中微污染物降解的特定作用–,强调了异质 Clhetero 和均质 CRs 和 Cl2 的重要性。
更新日期:2024-12-15
中文翻译:

通过电化学氧化工艺区分活性氯种类以去除含氯水中微污染物
电化学氧化过程 (EOP) 有望在水处理中降解微污染物,其中氯离子 (Cl–) 在水性系统中是不可避免的,从而导致 EOP/Cl– 系统。Cl 的氧化– 在阳极产生活性氯物质 (RCS),包括异质氯物质 (Clhetero)、均相游离有效氯 (FAC)、二氧化氯 (ClO2) 和氯自由基 (CR)。本研究开发了一种区分负责去除 EOP/Cl 中卡马西平的各种 RCS 的方法– 使用 RuO2/IrO2-Ti 阳极。与 EOP 相比,RCS 的形成显着增强了卡马西平在 EOP/Cl 中的降解–主要通过异质 Clhetero、均相分子氯 (Cl2) 和 CRs。特异性 RCS 对卡马西平降解的相对贡献在不同 pH 值、Cl– 浓度和电流密度下显著变化。当 pH 值从 5.3 增加到 10.0 时,10 mM Cl–,Clhetero 和 CRs 的相对贡献降低,而 Clhetero 在 pH 值为 7.0 和 10.0 时主导卡马西平降解。Cl2 在 pH 值为 5.3 时是卡马西平降解的优势物质,而在较高 pH 值下其作用显著降低。Cl– 浓度的增加增强了 Clhetero、Cl2 和 CRs 在 pH 5.3 和 18 mA/cm2 下的相对贡献。电流密度从 18 mA/cm2 增加到 39 mA/cm2 显着促进了 Clhetero 和 CR 在 pH 7.0 和 10 mM Cl 下的相对贡献–。本研究阐明了反应性物质对 EOP/Cl 中微污染物降解的特定作用–,强调了异质 Clhetero 和均质 CRs 和 Cl2 的重要性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号