当前位置:
X-MOL 学术
›
Sep. Purif. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Crystallization-based recovery of niobium compounds from alkaline liquor
Separation and Purification Technology ( IF 8.1 ) Pub Date : 2024-12-14 , DOI: 10.1016/j.seppur.2024.131085 Cássia Ribeiro Souza, Lucas Martins da Silva, Frederico Marques Penha, Sônia Denise Ferreira Rocha
Separation and Purification Technology ( IF 8.1 ) Pub Date : 2024-12-14 , DOI: 10.1016/j.seppur.2024.131085 Cássia Ribeiro Souza, Lucas Martins da Silva, Frederico Marques Penha, Sônia Denise Ferreira Rocha
|
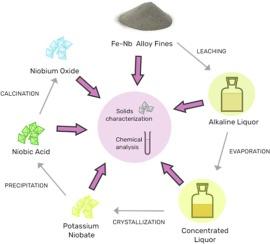
The consumption of niobium-based compounds has risen due to the broadening of the range of applications. Recent technological and industrial developments have increased the Nb demand, particularly in aviation, construction, electronics, communication, energy as well as the automotive, metallurgical and steel industries. In this regard, recovery of Nb from secondary sources is necessary to produce new compounds with high economic value. In this context, we used the Fe-Nb alloy fines, out of the commercial specifications, as a secondary source of niobium to produce an alkaline liquor to recover niobium compounds at relatively low temperatures and atmospheric pressure. Potassium niobate was obtained from the cooling crystallization of potassium alkaline liquor and was used as a precursor for producing niobic acid. The XRD and chemical analysis indicated that potassium niobate was formed in different crystalline phases – KNbO3 and K4 Nb6 O17 – with microscopy images showing isolated plate-like crystals. The chemical composition of the solid was 42.3 % of Nb, 24.7 % of K, 23.3 % of O and 9.7 % of other impurities. An amorphous niobic acid with residual potassium sulphate was obtained through precipitation from a potassium niobate solution with H2 SO4 0.5 mol/L. The chemical composition of niobic acid was 54.7 % of Nb, 11.7 % of K, 30.3 % of O and 2.4 % of S. The niobic acid was calcined at 900 °C for 5 h to produce niobium oxide. The XRD indicated the presence of a potassium niobate phase 61.2 % of Nb, 12.1 % of K, 32.6 % of O and 1.9 % of S. The presence of impurities in the solids is expected, due to its presence in the starting material. No impurity removal protocol was implemented.
中文翻译:

基于结晶的碱性液体中铌化合物的回收
由于应用范围的扩大,铌基化合物的消费量有所增加。最近的技术和工业发展增加了 Nb 需求,特别是在航空、建筑、电子、通信、能源以及汽车、冶金和钢铁行业。在这方面,从二级来源回收 Nb 对于生产具有高经济价值的新化合物是必要的。在这种情况下,我们使用商业规格之外的 Fe-Nb 合金细粉作为铌的辅助来源,以生产碱性液体,以在相对较低的温度和大气压下回收铌化合物。铌酸钾由钾碱性液冷却结晶而得,用作生产铌酸的前体。XRD 和化学分析表明,铌酸钾是在不同的晶相 KNbO3 和 K4Nb6O17 中形成的,显微镜图像显示孤立的板状晶体。固体的化学成分为 42.3% 的 Nb、24.7% 的 K、23.3% 的 O 和 9.7% 的其他杂质。从铌酸钾溶液中沉淀 H2SO4 0.5 mol/L,得到残留硫酸钾的无定形铌酸。铌酸的化学成分为 Nb 的 54.7 %、K 的 11.7 %、O 的 30.3 % 和 S 的 2.4 %。将铌酸在 900 °C 下煅烧 5 h,生成氧化铌。XRD 表明存在铌酸钾相,Nb为 61.2%,K 为 12.1%,O 为 32.6%,S 为 1.9%。由于起始材料中存在杂质,因此固体中存在杂质是意料之中的。未实施杂质去除方案。
更新日期:2024-12-14
中文翻译:

基于结晶的碱性液体中铌化合物的回收
由于应用范围的扩大,铌基化合物的消费量有所增加。最近的技术和工业发展增加了 Nb 需求,特别是在航空、建筑、电子、通信、能源以及汽车、冶金和钢铁行业。在这方面,从二级来源回收 Nb 对于生产具有高经济价值的新化合物是必要的。在这种情况下,我们使用商业规格之外的 Fe-Nb 合金细粉作为铌的辅助来源,以生产碱性液体,以在相对较低的温度和大气压下回收铌化合物。铌酸钾由钾碱性液冷却结晶而得,用作生产铌酸的前体。XRD 和化学分析表明,铌酸钾是在不同的晶相 KNbO3 和 K4Nb6O17 中形成的,显微镜图像显示孤立的板状晶体。固体的化学成分为 42.3% 的 Nb、24.7% 的 K、23.3% 的 O 和 9.7% 的其他杂质。从铌酸钾溶液中沉淀 H2SO4 0.5 mol/L,得到残留硫酸钾的无定形铌酸。铌酸的化学成分为 Nb 的 54.7 %、K 的 11.7 %、O 的 30.3 % 和 S 的 2.4 %。将铌酸在 900 °C 下煅烧 5 h,生成氧化铌。XRD 表明存在铌酸钾相,Nb为 61.2%,K 为 12.1%,O 为 32.6%,S 为 1.9%。由于起始材料中存在杂质,因此固体中存在杂质是意料之中的。未实施杂质去除方案。






























 京公网安备 11010802027423号
京公网安备 11010802027423号