当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cyclizative dearomative rearrangement of pyridines with isocyanates
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-12-14 , DOI: 10.1039/d4qo02111h Xing-Zi Li, Fang-Zhou Li, Zi-Qi Wang, Hua Wu
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-12-14 , DOI: 10.1039/d4qo02111h Xing-Zi Li, Fang-Zhou Li, Zi-Qi Wang, Hua Wu
|
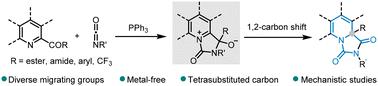
Dearomatization of pyridines is a robust synthetic method to access aza-heterocycles. Simultaneously, intermolecular cyclizative rearrangement is a recently developed strategy for efficiently constructing tetrasubstituted carbons. Here, we show that an effective integration of the dearomatization approach with strategic cyclizative rearrangement enables 2-acyl-substituted pyridines and their analogues with common isocyanates to undergo a tandem [3 + 2] heteroannulation, followed by an extensive 1,2-carbon shift, thus providing a straightforward access to readily functionalized bicyclohydantoins. Based on the promotion of organophosphorus, we have shown for the first time that different types of migrating groups, such as ester, amide, aryl and trifluoromethyl groups, are all well-tolerated in the same reaction.
中文翻译:

吡啶与异氰酸酯的环化脱氨基酸重排
吡啶的脱芳烃化是一种获得氮杂杂环的稳健合成方法。同时,分子间环化重排是最近开发的一种有效构建四取代碳的新策略。在这里,我们表明,脱芳烃化方法与策略性环化重排的有效整合使 2-酰基取代的吡啶及其类似物与常见的异氰酸酯发生串联 [3+2] 异质烷化,然后进行广泛的 1,2-碳转移,从而提供了易于官能化的双环乙内酰脲的直接途径。基于有机磷的促进,不同类型的迁移基团,如酯、酰胺、芳基和三氟甲基,首次在同一反应中都具有良好的耐受性。
更新日期:2024-12-14
中文翻译:

吡啶与异氰酸酯的环化脱氨基酸重排
吡啶的脱芳烃化是一种获得氮杂杂环的稳健合成方法。同时,分子间环化重排是最近开发的一种有效构建四取代碳的新策略。在这里,我们表明,脱芳烃化方法与策略性环化重排的有效整合使 2-酰基取代的吡啶及其类似物与常见的异氰酸酯发生串联 [3+2] 异质烷化,然后进行广泛的 1,2-碳转移,从而提供了易于官能化的双环乙内酰脲的直接途径。基于有机磷的促进,不同类型的迁移基团,如酯、酰胺、芳基和三氟甲基,首次在同一反应中都具有良好的耐受性。






























 京公网安备 11010802027423号
京公网安备 11010802027423号