当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of 1,4,2-Diazaphospholidine-3,5-diones Using Na(OCP) as the “P” Source
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-12-13 , DOI: 10.1021/acs.joc.4c02259 Yao Chai, Ya-Ling Tian, Wen-Bo Xu, Bo Yang, Zhi-Bin Wang, Dong-Ping Chen, Xi-Cun Wang, Zheng-Jun Quan
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-12-13 , DOI: 10.1021/acs.joc.4c02259 Yao Chai, Ya-Ling Tian, Wen-Bo Xu, Bo Yang, Zhi-Bin Wang, Dong-Ping Chen, Xi-Cun Wang, Zheng-Jun Quan
|
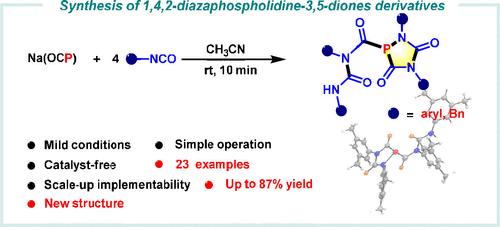
A refined synthesis of 1,4,2-diazaphospholidine-3,5-dione derivatives was achieved through a cyclization reaction involving Na(OCP) and isocyanates. Na(OCP) was demonstrated to be a relatively stable and safe source of phosphorus, enabling the production of diverse 1,4,2-diazaphospholidine-3,5-dione derivatives with high yields. The reaction proceeds efficiently under catalyst-free and mild conditions. Both experimental findings and density functional theory calculations have elucidated that the process involves a crucial step of carbon monoxide elimination, which provides deeper insight into the reaction mechanism.
中文翻译:

使用 Na(OCP) 作为“P”源合成 1,4,2-二氮杂膦-3,5-二酮
通过涉及 Na (OCP) 和异氰酸酯的环化反应实现了 1,4,2-二氮杂膦-3,5-二酮衍生物的精制合成。Na (OCP) 被证明是一种相对稳定和安全的磷来源,能够以高产率生产多种 1,4,2-二氮杂磷酸-3,5-二酮衍生物。反应在无催化剂和温和条件下高效进行。实验结果和密度泛函理论计算都阐明了该过程涉及一氧化碳消除的关键步骤,这为反应机理提供了更深入的见解。
更新日期:2024-12-13
中文翻译:

使用 Na(OCP) 作为“P”源合成 1,4,2-二氮杂膦-3,5-二酮
通过涉及 Na (OCP) 和异氰酸酯的环化反应实现了 1,4,2-二氮杂膦-3,5-二酮衍生物的精制合成。Na (OCP) 被证明是一种相对稳定和安全的磷来源,能够以高产率生产多种 1,4,2-二氮杂磷酸-3,5-二酮衍生物。反应在无催化剂和温和条件下高效进行。实验结果和密度泛函理论计算都阐明了该过程涉及一氧化碳消除的关键步骤,这为反应机理提供了更深入的见解。






























 京公网安备 11010802027423号
京公网安备 11010802027423号