当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Anionic Oxidation Activity/Stability Regulated by Transition Metals in Multimetallic (Oxy)hydroxides for Alkaline Water Oxidation
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-12-12 , DOI: 10.1021/acscatal.4c03718 Shuhao Wang, Kamran Dastafkan, Sicheng Wu, Qian Sun, Chengli Rong, Dazhi Yao, Chuan Zhao
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-12-12 , DOI: 10.1021/acscatal.4c03718 Shuhao Wang, Kamran Dastafkan, Sicheng Wu, Qian Sun, Chengli Rong, Dazhi Yao, Chuan Zhao
|
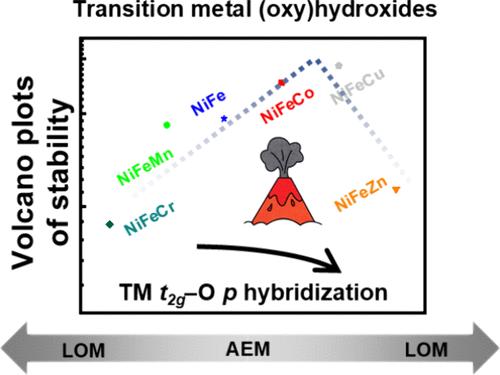
Transition metal (oxy)hydroxides are currently the benchmark materials for the oxygen evolution reaction (OER) in alkaline media. Superior activity can be achieved due to the anionic redox mechanism of transition metal (oxy)hydroxides, which enables the OER to proceed with the lattice oxygen pathway beyond the conventional adsorbate evolution mechanism. Although it is widely accepted that lattice oxygen oxidation stems from the high energy nonbonding oxygen states, which is mainly determined by the π-type hybridization between metal and oxygen orbitals, how to regulate the lattice oxygen oxidation mechanism remains a challenge. Here, the lattice oxygen oxidation in the benchmark NiFe (oxy)hydroxides is investigated by introducing 3d transition-metal dopants. We discover that the hybridization between metal t2g orbitals and oxygen p orbitals is essential for the energy level of oxygen states by density functional theory and is regulated by the number and energy level of transition metal d electrons. We propose that constratedly strong or weak hybridization between transition metal t2g and oxygen p orbitals is key to activating lattice oxygen oxidation. Supported by electrochemical tests and spectroscopic characterizations, the regulation of the anionic oxidation in transition metal (oxy)hydroxides will enable the control of cumulative lattice oxygen reactions for developing efficient and robust oxygen evolution electrocatalysts.
中文翻译:

多金属(氧)氢氧化物中过渡金属对碱性水氧化的阴离子氧化活性/稳定性的调节
过渡金属(氧)氢氧化物是目前碱性介质中析氧反应 (OER) 的基准材料。由于过渡金属(氧)氢氧化物的阴离子氧化还原机制,可以实现卓越的活性,这使得 OER 能够超越传统的吸附物析出机制进行晶格氧途径。尽管人们普遍认为晶格氧氧化源于高能非键合氧态,这主要由金属和氧轨道之间的π型杂化决定,但如何调控晶格氧氧化机制仍然是一个挑战。在这里,通过引入 3d 过渡金属掺杂剂来研究基准 NiFe(氧)氢氧化物中的晶格氧氧化。我们发现金属 t2g 轨道和氧 p 轨道之间的杂化对于密度泛函理论对氧态的能级至关重要,并受过渡金属 d 电子的数量和能级的调节。我们提出,过渡金属 t2g 和氧 p 轨道之间的强或弱杂化是激活晶格氧氧化的关键。在电化学测试和光谱表征的支持下,过渡金属(氧)氢氧化物中阴离子氧化的调节将能够控制累积晶格氧反应,从而开发高效和稳定的析氧电催化剂。
更新日期:2024-12-13
中文翻译:

多金属(氧)氢氧化物中过渡金属对碱性水氧化的阴离子氧化活性/稳定性的调节
过渡金属(氧)氢氧化物是目前碱性介质中析氧反应 (OER) 的基准材料。由于过渡金属(氧)氢氧化物的阴离子氧化还原机制,可以实现卓越的活性,这使得 OER 能够超越传统的吸附物析出机制进行晶格氧途径。尽管人们普遍认为晶格氧氧化源于高能非键合氧态,这主要由金属和氧轨道之间的π型杂化决定,但如何调控晶格氧氧化机制仍然是一个挑战。在这里,通过引入 3d 过渡金属掺杂剂来研究基准 NiFe(氧)氢氧化物中的晶格氧氧化。我们发现金属 t2g 轨道和氧 p 轨道之间的杂化对于密度泛函理论对氧态的能级至关重要,并受过渡金属 d 电子的数量和能级的调节。我们提出,过渡金属 t2g 和氧 p 轨道之间的强或弱杂化是激活晶格氧氧化的关键。在电化学测试和光谱表征的支持下,过渡金属(氧)氢氧化物中阴离子氧化的调节将能够控制累积晶格氧反应,从而开发高效和稳定的析氧电催化剂。






























 京公网安备 11010802027423号
京公网安备 11010802027423号