当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electroreductive Cross-Coupling Reactions: Carboxylation, Deuteration, and Alkylation
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2024-12-13 , DOI: 10.1021/acs.accounts.4c00652 Pengfei Li, Yanwei Wang, Hanying Zhao, Youai Qiu
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2024-12-13 , DOI: 10.1021/acs.accounts.4c00652 Pengfei Li, Yanwei Wang, Hanying Zhao, Youai Qiu
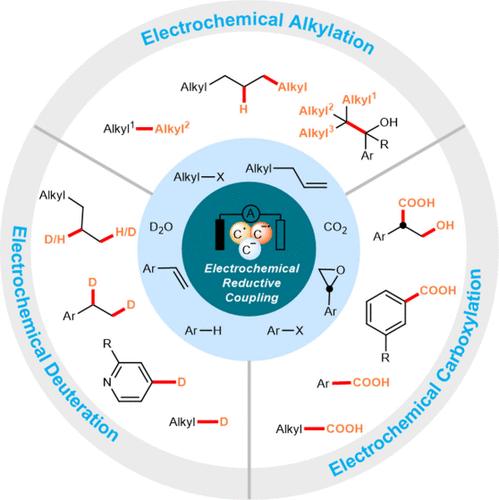
|
Electrochemistry has been used as a tool to drive chemical reactions for more than two centuries. With the help of an electrode and a power source, chemists are provided with a system whose potential can be precisely dialed in. The theoretically infinite redox range renders electrochemistry capable of oxidizing or reducing some of the most tenacious compounds. Indeed, electroreduction offers an alternative to generating highly active intermediates from electrophiles (e.g., halides, alkenes, etc.) in organic synthesis, which can be untouchable with traditional reduction methods. Meanwhile, the reductive coupling reactions are extensively utilized in both industrial and academic settings due to their ability to swiftly, accurately, and effectively construct C–C and C–X bonds, which present innovative approaches for synthesizing complex molecules. Nonetheless, its application is constrained by several inherent limitations: (a) the requirement for stoichiometric quantities of reducing agents, (b) scarce activation strategies for inert substrates with high reduction potentials, (c) incomplete mechanistic elucidation, and (d) challenges in the isolation of intermediates. The merging of electrochemistry and reductive coupling represents an attractive approach to address the above limitations in organic synthesis and has seen increasing use in the synthetic community over the past few years.
中文翻译:

电还原交叉偶联反应:羧化、氘化和烷基化
两个多世纪以来,电化学一直被用作驱动化学反应的工具。在电极和电源的帮助下,化学家们得到了一个可以精确调节其潜力的系统。理论上无限的氧化还原范围使电化学能够氧化或还原一些最顽固的化合物。事实上,电还原为有机合成中从亲电试剂(例如卤化物、烯烃等)生成高活性中间体提供了一种替代方案,这是传统还原方法无法实现的。同时,还原偶联反应因其能够快速、准确和有效地构建 C-C 和 C-X 键而在工业和学术环境中得到广泛应用,这为合成复杂分子提供了创新方法。尽管如此,其应用受到几个固有限制的限制:(a) 对还原剂化学计量量的要求,(b) 具有高还原电位的惰性底物的稀缺活化策略,(c) 不完整的机理阐明,以及 (d) 中间体分离的挑战。电化学和还原耦合的合并代表了解决有机合成中上述限制的一种有吸引力的方法,并且在过去几年中在合成社区中的应用越来越多。
更新日期:2024-12-13
中文翻译:

电还原交叉偶联反应:羧化、氘化和烷基化
两个多世纪以来,电化学一直被用作驱动化学反应的工具。在电极和电源的帮助下,化学家们得到了一个可以精确调节其潜力的系统。理论上无限的氧化还原范围使电化学能够氧化或还原一些最顽固的化合物。事实上,电还原为有机合成中从亲电试剂(例如卤化物、烯烃等)生成高活性中间体提供了一种替代方案,这是传统还原方法无法实现的。同时,还原偶联反应因其能够快速、准确和有效地构建 C-C 和 C-X 键而在工业和学术环境中得到广泛应用,这为合成复杂分子提供了创新方法。尽管如此,其应用受到几个固有限制的限制:(a) 对还原剂化学计量量的要求,(b) 具有高还原电位的惰性底物的稀缺活化策略,(c) 不完整的机理阐明,以及 (d) 中间体分离的挑战。电化学和还原耦合的合并代表了解决有机合成中上述限制的一种有吸引力的方法,并且在过去几年中在合成社区中的应用越来越多。






























 京公网安备 11010802027423号
京公网安备 11010802027423号