当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Insights into the Roles of Different Iron Species on Zeolites for N2O Selective Catalytic Reduction by CO
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2024-12-13 , DOI: 10.1021/acs.est.4c06924 Yunshuo Wu, Xuanhao Wu, Jie Fan, Haiqiang Wang, Zhongbiao Wu
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2024-12-13 , DOI: 10.1021/acs.est.4c06924 Yunshuo Wu, Xuanhao Wu, Jie Fan, Haiqiang Wang, Zhongbiao Wu
|
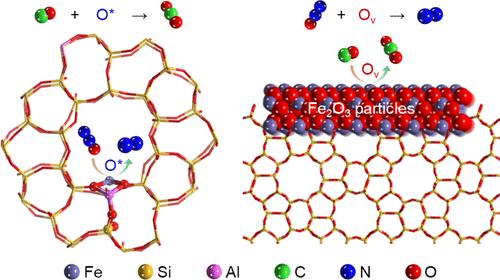
Iron zeolites are promising candidates for mitigating nitrous oxide (N2O), a potent greenhouse gas and contributor to stratospheric ozone destruction. However, the atomic-level mechanisms by which different iron species, including isolated sites, clusters, and particles, participate in N2O decomposition in the presence of CO still remain poorly understood, which hinders the application of the reaction in practical technology. Herein, through experiments and density functional theory (DFT) calculations, we identified that isolated iron sites were active for N2O activation to generate adsorbed O* species, which readily reacted with CO following the Eley–Rideal (E-R) mechanism. In contrast, Fe2O3 particles exhibited a different reaction pathway, directly reacting with CO to generate oxygen vacancies (Ov), which could efficiently dissociate N2O following the Mars-van Krevelen (MvK) mechanism. Moreover, the transformation of iron oxide clusters into undercoordinated FeOx species by CO was also revealed through various techniques, such as CO-temperature-programmed reduction (TPR), and ab initio molecular dynamics (AIMD) simulations. Our study provides deeper insights into the roles of different iron species in N2O-SCR by CO, and is anticipated to facilitate the understanding of multicomponent catalysis and the design of efficient iron-containing catalysts for practical applications.
中文翻译:

深入了解不同铁种类对沸石对 CO N2O 选择性催化还原的作用
铁沸石是减轻一氧化二氮 (N2O) 的有前途的候选者,一氧化二氮是一种强效的温室气体,是平流层臭氧破坏的贡献者。然而,在 CO 存在下,不同铁种类(包括孤立位点、团簇和颗粒)参与 N2O 分解的原子级机制仍然知之甚少,这阻碍了该反应在实际技术中的应用。在此,通过实验和密度泛函理论 (DFT) 计算,我们确定分离的铁位点对 N2O 激活具有活性,以产生吸附的 O* 物质,这些物质很容易按照 Eley-Rideal (E-R) 机制与 CO 反应。相比之下,Fe2O3 颗粒表现出不同的反应途径,直接与 CO 反应产生氧空位 (Ov),这可以按照 Mars-van Krevelen (MvK) 机制有效地解离 N2O。此外,还通过各种技术揭示了 CO 将氧化铁簇转化为配位不足的 FeOx 物质,例如 CO 程序升温还原 (TPR) 和从头计算分子动力学 (AIMD) 模拟。我们的研究为不同铁种类在 CO 的 N2O-SCR 中的作用提供了更深入的见解,并有望促进对多组分催化的理解和为实际应用设计高效的含铁催化剂。
更新日期:2024-12-13
中文翻译:

深入了解不同铁种类对沸石对 CO N2O 选择性催化还原的作用
铁沸石是减轻一氧化二氮 (N2O) 的有前途的候选者,一氧化二氮是一种强效的温室气体,是平流层臭氧破坏的贡献者。然而,在 CO 存在下,不同铁种类(包括孤立位点、团簇和颗粒)参与 N2O 分解的原子级机制仍然知之甚少,这阻碍了该反应在实际技术中的应用。在此,通过实验和密度泛函理论 (DFT) 计算,我们确定分离的铁位点对 N2O 激活具有活性,以产生吸附的 O* 物质,这些物质很容易按照 Eley-Rideal (E-R) 机制与 CO 反应。相比之下,Fe2O3 颗粒表现出不同的反应途径,直接与 CO 反应产生氧空位 (Ov),这可以按照 Mars-van Krevelen (MvK) 机制有效地解离 N2O。此外,还通过各种技术揭示了 CO 将氧化铁簇转化为配位不足的 FeOx 物质,例如 CO 程序升温还原 (TPR) 和从头计算分子动力学 (AIMD) 模拟。我们的研究为不同铁种类在 CO 的 N2O-SCR 中的作用提供了更深入的见解,并有望促进对多组分催化的理解和为实际应用设计高效的含铁催化剂。

































 京公网安备 11010802027423号
京公网安备 11010802027423号