当前位置:
X-MOL 学术
›
Cell Metab.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
FcRn-dependent IgG accumulation in adipose tissue unmasks obesity pathophysiology
Cell Metabolism ( IF 27.7 ) Pub Date : 2024-12-13 , DOI: 10.1016/j.cmet.2024.11.001 Lexiang Yu, Yong Xiao Yang, Zhen Gong, Qianfen Wan, Yifei Du, Qiuzhong Zhou, Yang Xiao, Tarik Zahr, Zhaobin Wang, Zhewei Yu, Kangkang Yang, Jinyang Geng, Susan K. Fried, Jing Li, Rebecca A. Haeusler, Kam W. Leong, Lin Bai, Yingjie Wu, Lei Sun, Pan Wang, Li Qiang
Cell Metabolism ( IF 27.7 ) Pub Date : 2024-12-13 , DOI: 10.1016/j.cmet.2024.11.001 Lexiang Yu, Yong Xiao Yang, Zhen Gong, Qianfen Wan, Yifei Du, Qiuzhong Zhou, Yang Xiao, Tarik Zahr, Zhaobin Wang, Zhewei Yu, Kangkang Yang, Jinyang Geng, Susan K. Fried, Jing Li, Rebecca A. Haeusler, Kam W. Leong, Lin Bai, Yingjie Wu, Lei Sun, Pan Wang, Li Qiang
|
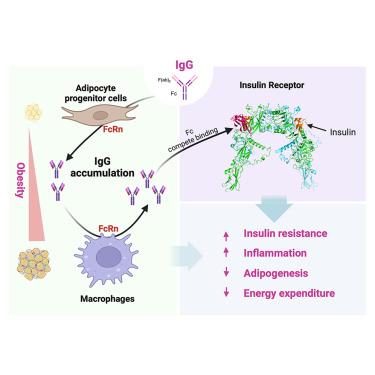
Immunoglobulin G (IgG) is traditionally recognized as a plasma protein that neutralizes antigens for immune defense. However, our research demonstrates that IgG predominantly accumulates in adipose tissue during obesity development, triggering insulin resistance and macrophage infiltration. This accumulation is governed by neonatal Fc receptor (FcRn)-dependent recycling, orchestrated in adipose progenitor cells and macrophages during the early and late stages of diet-induced obesity (DIO), respectively. Targeting FcRn abolished IgG accumulation and rectified insulin resistance and metabolic degeneration in DIO. By integrating artificial intelligence (AI) modeling with in vivo and in vitro experimental models, we unexpectedly uncovered an interaction between IgG’s Fc-CH3 domain and the insulin receptor's ectodomain. This interaction hinders insulin binding, consequently obstructing insulin signaling and adipocyte functions. These findings unveil adipose IgG accumulation as a driving force in obesity pathophysiology, providing a novel therapeutic strategy to tackle metabolic dysfunctions.
中文翻译:

脂肪组织中 FcRn 依赖性 IgG 积累揭示了肥胖的病理生理学
免疫球蛋白 G (IgG) 传统上被认为是一种血浆蛋白,可中和抗原以进行免疫防御。然而,我们的研究表明,IgG 主要在肥胖发展过程中积累在脂肪组织中,引发胰岛素抵抗和巨噬细胞浸润。这种积累由新生儿 Fc 受体 (FcRn) 依赖性循环控制,分别在饮食诱导的肥胖 (DIO) 的早期和晚期在脂肪祖细胞和巨噬细胞中精心编排。靶向 FcRn 消除了 IgG 积累并纠正了 DIO 中的胰岛素抵抗和代谢变性。通过将人工智能 (AI) 建模与体内 和 体外实验模型相结合,我们出乎意料地发现了 IgG 的 Fc-CH3 结构域与胰岛素受体的胞外结构域之间的相互作用。这种相互作用阻碍了胰岛素结合,从而阻碍了胰岛素信号传导和脂肪细胞功能。这些发现揭示了脂肪 IgG 积累是肥胖病理生理学的驱动力,为解决代谢功能障碍提供了一种新的治疗策略。
更新日期:2024-12-13
中文翻译:

脂肪组织中 FcRn 依赖性 IgG 积累揭示了肥胖的病理生理学
免疫球蛋白 G (IgG) 传统上被认为是一种血浆蛋白,可中和抗原以进行免疫防御。然而,我们的研究表明,IgG 主要在肥胖发展过程中积累在脂肪组织中,引发胰岛素抵抗和巨噬细胞浸润。这种积累由新生儿 Fc 受体 (FcRn) 依赖性循环控制,分别在饮食诱导的肥胖 (DIO) 的早期和晚期在脂肪祖细胞和巨噬细胞中精心编排。靶向 FcRn 消除了 IgG 积累并纠正了 DIO 中的胰岛素抵抗和代谢变性。通过将人工智能 (AI) 建模与体内 和 体外实验模型相结合,我们出乎意料地发现了 IgG 的 Fc-CH3 结构域与胰岛素受体的胞外结构域之间的相互作用。这种相互作用阻碍了胰岛素结合,从而阻碍了胰岛素信号传导和脂肪细胞功能。这些发现揭示了脂肪 IgG 积累是肥胖病理生理学的驱动力,为解决代谢功能障碍提供了一种新的治疗策略。






























 京公网安备 11010802027423号
京公网安备 11010802027423号