当前位置:
X-MOL 学术
›
Tetrahedron
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of bis-Quaternary Centers at the α-Positions of Cyclohexanones via Copper(I)-Catalyzed Claisen Rearrangement: The Substituent Effect from the Opposing α-Quaternary Center on Diastereoselectivity
Tetrahedron ( IF 2.1 ) Pub Date : 2024-12-12 , DOI: 10.1016/j.tet.2024.134415 Satish Chandra Philkhana, Joshua A. Malone, Estefania Armendariz-Gonzalez, Adi Saputra, Jacob R. Stepherson, Frank R. Fronczek, Rendy Kartika
Tetrahedron ( IF 2.1 ) Pub Date : 2024-12-12 , DOI: 10.1016/j.tet.2024.134415 Satish Chandra Philkhana, Joshua A. Malone, Estefania Armendariz-Gonzalez, Adi Saputra, Jacob R. Stepherson, Frank R. Fronczek, Rendy Kartika
|
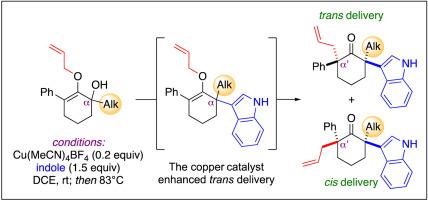
We report the synthesis of bis-quaternary centers at the α-positions of cyclohexanones. In this chemistry, ionization of an α-hydroxy allyl vinyl ether with Cu(MeCN)4BF4 catalyst at room temperature formed an allyloxy allyl cation that was captured by indole in a regioselective manner to create an α-quaternary center. Upon heating, the Claisen rearrangement occurred in situ to produce a second quaternary center at the opposing α’-position, thereby furnishing the targeted bis-quaternary center motif. Studies in this article examined the alkyl substituents at the α-carbon and their effect on diastereoselectivity of the Claisen rearrangement. Interestingly, the copper catalyst enhanced the trans delivery of the allyl group with respect to the indole versus the background diastereoselectivity of the uncatalyzed reaction.
中文翻译:

通过铜(I)催化的Claisen重排合成环己酮α位的双四元中心:对立α四元中心对非对映选择性的取代基效应
我们报道了环己酮 α 位双四元中心的合成。在该化学中,α-羟基烯丙基乙烯基醚与 Cu(MeCN)4BF4 催化剂在室温下电离形成烯丙氧基烯丙基阳离子,该阳离子被吲哚以区域选择性方式捕获,形成α-季铵中心。加热后,Claisen 重排在原位发生,在相反的 α'-位置产生第二个四元中心,从而提供目标双四元中心基序。本文的研究检查了 α-碳处的烷基取代基及其对 Claisen 重排的非对映选择性的影响。有趣的是,与未催化反应的背景非对映选择性相比,铜催化剂增强了烯丙基相对于吲哚的反式递送。
更新日期:2024-12-13
中文翻译:

通过铜(I)催化的Claisen重排合成环己酮α位的双四元中心:对立α四元中心对非对映选择性的取代基效应
我们报道了环己酮 α 位双四元中心的合成。在该化学中,α-羟基烯丙基乙烯基醚与 Cu(MeCN)4BF4 催化剂在室温下电离形成烯丙氧基烯丙基阳离子,该阳离子被吲哚以区域选择性方式捕获,形成α-季铵中心。加热后,Claisen 重排在原位发生,在相反的 α'-位置产生第二个四元中心,从而提供目标双四元中心基序。本文的研究检查了 α-碳处的烷基取代基及其对 Claisen 重排的非对映选择性的影响。有趣的是,与未催化反应的背景非对映选择性相比,铜催化剂增强了烯丙基相对于吲哚的反式递送。































 京公网安备 11010802027423号
京公网安备 11010802027423号