当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design and synthesis of novel 3,7-dihydro-1H-purine-2,6-diones as DPP-4 inhibitors: An in silico, in vitro and in vivo approach
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2024-12-12 , DOI: 10.1016/j.ejmech.2024.117160 Priya Bisht, Arka Bhattacharya, Anubroto Pal, Rajveer Singh, Sant Kumar Verma
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2024-12-12 , DOI: 10.1016/j.ejmech.2024.117160 Priya Bisht, Arka Bhattacharya, Anubroto Pal, Rajveer Singh, Sant Kumar Verma
|
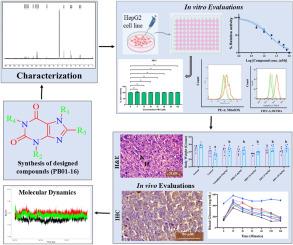
The inhibition of enzyme DPP-4 is pivotal for targeting type 2 diabetes mellitus (DM). The study introduces two series of novel 1,3-dimethyl-3,7-dihydro-1H -purine-2,6-diones derivatives (PB01-PB10) and 3,7-dihydro-1H -purine-2,6-diones compounds (PB11-PB16) were developed using linagliptin scaffold. Sixteen derivatives were synthesized and screened in vitro against DPP-4, revealing IC50 ranging from 15.66 ± 2.546 to 28.45 ± 4.441 nM. Compounds PB01 and PB11 demonstrated high potency comparable to reference standard linagliptin (IC50 = 15.66 ± 2.546, 16.16 ± 1.214, 15.37 ± 2.481 nM, respectively). Further studies showed that the compound possesses negligible cytotoxicity up to 100 μM concentration. A high glucose-induced DPP-4 upregulation model was further utilized to assess the protective effect of PB01 and PB11, and their efficacy was compared with linagliptin. PB01 and PB11 showed comparable protective effects against high glucose-induced ROS generation and mitochondrial superoxide production, and the compounds also effectively reduced the DPP-4 cellular expression. The in vivo anti-diabetic efficacy, effect on change in body weight, and OGTT due to PB01 and PB11 treatments were evaluated using the STZ-Nicotinamide-induced experimental model of diabetes in mice. Post induction of diabetes, the periodic estimation of blood serum glucose levels reveals that treatment with PB01 and PB11 decreased the high blood serum glucose levels in both acute and chronic studies. The expressions of DPP-4 were observed by IHC, Flowcytometry, and RT-qPCR. The docked complexes of both compounds, along with the standard drug linagliptin, were subjected to molecular dynamics simulation for 140ns to evaluate the complexes' stability and binding affinity.
中文翻译:

新型 3,7-二氢-1H-嘌呤-2,6-二酮作为 DPP-4 抑制剂的设计与合成:一种计算机、体外和体内方法
抑制 DPP-4 酶对于靶向 2 型糖尿病 (DM) 至关重要。该研究介绍了两个系列的新型 1,3-二甲基-3,7-二氢-1H-嘌呤-2,6-二酮衍生物 (PB01-PB10) 和 3,7-二氢-1H-嘌呤-2,6-二酮化合物 (PB11-PB16) 是使用利格列汀支架开发的。合成了 16 种衍生物并在体外针对 DPP-4 进行了筛选,显示 IC50 范围为 15.66 ± 2.546 至 28.45 ± 4.441 nM。化合物 PB01 和 PB11 表现出与参考标准利格列汀相当的高效力 (IC50 = 15.66 ± 2.546、16.16 ± 1.214、15.37 ± 2.481 nM)。进一步的研究表明,该化合物在高达 100 μM 浓度时具有可忽略不计的细胞毒性。进一步采用高葡萄糖诱导的 DPP-4 上调模型评价 PB01 和 PB11 的保护作用,并比较它们与利格列汀的疗效。PB01 和 PB11 对高葡萄糖诱导的 ROS 生成和线粒体超氧化物的产生表现出相当的保护作用,并且这些化合物还有效降低了 DPP-4 细胞表达。使用 STZ-烟酰胺诱导的小鼠糖尿病实验模型评价 PB01 和 PB11 治疗引起的体内抗糖尿病疗效、对体重变化的影响以及 OGTT。糖尿病诱导后,血血清葡萄糖水平的定期估计表明,在急性和慢性研究中,PB01 和 PB11 治疗降低了高血血清葡萄糖水平。IHC 、 流式细胞术 和 RT-qPCR 观察 DPP-4 的表达。将两种化合物的对接复合物以及标准药物利格列汀进行 140 ns 的分子动力学模拟,以评估复合物的稳定性和结合亲和力。
更新日期:2024-12-12
中文翻译:

新型 3,7-二氢-1H-嘌呤-2,6-二酮作为 DPP-4 抑制剂的设计与合成:一种计算机、体外和体内方法
抑制 DPP-4 酶对于靶向 2 型糖尿病 (DM) 至关重要。该研究介绍了两个系列的新型 1,3-二甲基-3,7-二氢-1H-嘌呤-2,6-二酮衍生物 (PB01-PB10) 和 3,7-二氢-1H-嘌呤-2,6-二酮化合物 (PB11-PB16) 是使用利格列汀支架开发的。合成了 16 种衍生物并在体外针对 DPP-4 进行了筛选,显示 IC50 范围为 15.66 ± 2.546 至 28.45 ± 4.441 nM。化合物 PB01 和 PB11 表现出与参考标准利格列汀相当的高效力 (IC50 = 15.66 ± 2.546、16.16 ± 1.214、15.37 ± 2.481 nM)。进一步的研究表明,该化合物在高达 100 μM 浓度时具有可忽略不计的细胞毒性。进一步采用高葡萄糖诱导的 DPP-4 上调模型评价 PB01 和 PB11 的保护作用,并比较它们与利格列汀的疗效。PB01 和 PB11 对高葡萄糖诱导的 ROS 生成和线粒体超氧化物的产生表现出相当的保护作用,并且这些化合物还有效降低了 DPP-4 细胞表达。使用 STZ-烟酰胺诱导的小鼠糖尿病实验模型评价 PB01 和 PB11 治疗引起的体内抗糖尿病疗效、对体重变化的影响以及 OGTT。糖尿病诱导后,血血清葡萄糖水平的定期估计表明,在急性和慢性研究中,PB01 和 PB11 治疗降低了高血血清葡萄糖水平。IHC 、 流式细胞术 和 RT-qPCR 观察 DPP-4 的表达。将两种化合物的对接复合物以及标准药物利格列汀进行 140 ns 的分子动力学模拟,以评估复合物的稳定性和结合亲和力。

































 京公网安备 11010802027423号
京公网安备 11010802027423号