当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Palladium-catalyzed three-component annulation reaction involving multiple C–H activation
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-12-13 , DOI: 10.1039/d4qo01857e Shuai Yang, Xiang Zuo, Yanghui Zhang
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-12-13 , DOI: 10.1039/d4qo01857e Shuai Yang, Xiang Zuo, Yanghui Zhang
|
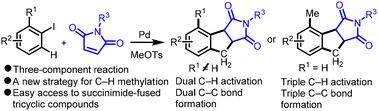
The Pd-catalyzed ring-forming reaction via multiple C–H activation provides an efficient strategy to access cyclic ring systems. The current reactions are primarily restricted to single- and two-component reactions. Herein, we report a ring-forming reaction via palladium-catalyzed three-component multiple C–H activation. Using TsOMe as the methylating reagent, aryl iodides undergo maleimide-relayed C–H methylation. Subsequent cyclization via C(sp3)–H activation forms succinimide-fused tricyclic scaffolds. Depending on aryl iodides, the reaction involves dual or triple C–H activation to form two or three new C–C bonds. The reaction represents a new strategy for C–H methylation and offers a new synthetic method using simple and readily available substrates for succinimide-fused tricyclic scaffolds, which are crucial structural motifs found widely in organic compounds with diverse biological activities.
中文翻译:

钯催化的三组分环化反应,涉及多次 C-H 活化
通过多次 C-H 活化的 Pd 催化的成环反应提供了一种进入环系统的有效策略。当前反应主要限于单组分和双组分反应。在此,我们报道了通过钯催化的三组分多重 C-H 活化的成环反应。使用 TsOMe 作为甲基化试剂,芳基碘化物发生马来酰亚胺中继的 C-H 甲基化。随后通过 C(sp3)-H 活化环化形成琥珀酰亚胺熔合的三环支架。根据芳基碘化物的不同,该反应涉及双重或三重 C-H 活化,以形成两个或三个新的 C-C 键。该反应代表了 C-H 甲基化的新策略,并提供了一种新的合成方法,使用简单易得的底物用于琥珀酰亚胺熔融的三环支架,这是广泛存在于具有不同生物活性的有机化合物中的关键结构基序。
更新日期:2024-12-13
中文翻译:

钯催化的三组分环化反应,涉及多次 C-H 活化
通过多次 C-H 活化的 Pd 催化的成环反应提供了一种进入环系统的有效策略。当前反应主要限于单组分和双组分反应。在此,我们报道了通过钯催化的三组分多重 C-H 活化的成环反应。使用 TsOMe 作为甲基化试剂,芳基碘化物发生马来酰亚胺中继的 C-H 甲基化。随后通过 C(sp3)-H 活化环化形成琥珀酰亚胺熔合的三环支架。根据芳基碘化物的不同,该反应涉及双重或三重 C-H 活化,以形成两个或三个新的 C-C 键。该反应代表了 C-H 甲基化的新策略,并提供了一种新的合成方法,使用简单易得的底物用于琥珀酰亚胺熔融的三环支架,这是广泛存在于具有不同生物活性的有机化合物中的关键结构基序。






























 京公网安备 11010802027423号
京公网安备 11010802027423号