Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synchrotron-based infrared microspectroscopy unveils the biomolecular response of healthy and tumour cell lines to neon minibeam radiation therapy
Analyst ( IF 3.6 ) Pub Date : 2024-12-13 , DOI: 10.1039/d4an01038h R. González-Vegas, O. Seksek, A. Bertho, J. Bergs, R. Hirayama, T. Inaniwa, N. Matsufuji, T. Shimokawa, Y. Prezado, I. Yousef, I. Martínez-Rovira
Analyst ( IF 3.6 ) Pub Date : 2024-12-13 , DOI: 10.1039/d4an01038h R. González-Vegas, O. Seksek, A. Bertho, J. Bergs, R. Hirayama, T. Inaniwa, N. Matsufuji, T. Shimokawa, Y. Prezado, I. Yousef, I. Martínez-Rovira
|
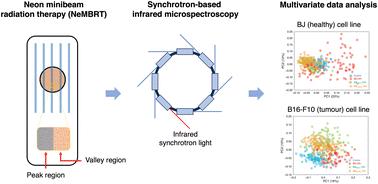
Radioresistant tumours remain complex to manage with current radiotherapy (RT) techniques. Heavy ion beams were proposed for their treatment given their advantageous radiobiological properties. However, previous studies with patients resulted in serious adverse effects in the surrounding healthy tissues. Heavy ion RT could therefore benefit from the tissue-sparing effects of minibeam radiation therapy (MBRT). To investigate the potential of this combination, here we assessed the biochemical response to neon MBRT (NeMBRT) through synchrotron-based Fourier transform infrared microspectroscopy (SR-FTIRM). Healthy (BJ) and tumour (B16-F10) cell lines were subjected to seamless (broad beam) neon RT (NeBB) and NeMBRT at HIMAC. SR-FTIRM measurements were conducted at the MIRAS beamline of ALBA Synchrotron. Principal component analysis (PCA) permitted to assess the biochemical effects after the irradiations and 24 hours post-irradiation for the different RT modalities and doses. For the healthy cells, NeMBRT resulted in the most dissimilar spectral signatures from non-irradiated cells early after irradiations, mainly due to protein conformational modifications. Nevertheless, most of the damage appeared to recover one day post-RT; conversely, protein- and nucleic acid-related IR bands were strongly affected by NeBB 24 hours after treatment, suggesting superior oxidative damage and nucleic acid degradation. Tumour cells appeared to be less sensitive to NeBB than to NeMBRT shortly after RT. Still, after one day, both NeBB and the high-dose NeMBRT regions yielded important spectral modifications, suggestive of cell death processes, protein oxidation or oxidative stress. Lipid-associated spectral changes, especially due to the NeBB and NeMBRT peak groups for the tumour cell line, were consistent with reactive oxygen species attacks.
中文翻译:

基于同步加速器的红外显微光谱揭示了健康和肿瘤细胞系对氖微束放射治疗的生物分子反应
使用当前的放疗 (RT) 技术管理放射耐药性肿瘤仍然很复杂。鉴于其有利的放射生物学特性,建议使用重离子束进行治疗。然而,先前对患者的研究对周围健康组织产生了严重的不利影响。因此,重粒子线 RT 可能受益于微束放射治疗 (MBRT) 的组织保留效应。为了研究这种组合的潜力,我们通过基于同步加速器的傅里叶变换红外显微光谱 (SR-FTIRM) 评估了对氖 MBRT (NeMBRT) 的生化反应。在 HIMAC 对健康 (BJ) 和肿瘤 (B16-F10) 细胞系进行无缝(宽光束)氖 RT (NeBB) 和 NeMBRT 检测。SR-FTIRM 测量在 ALBA 同步加速器的 MIRAS 光束线上进行。允许主成分分析 (PCA) 评估不同 RT 方式和剂量的照射后和照射后 24 小时的生化影响。对于健康细胞,NeMBRT 在照射后早期导致与未照射细胞最不同的光谱特征,主要是由于蛋白质构象修饰。尽管如此,大部分损伤似乎在 RT 后一天恢复了;相反,蛋白质和核酸相关的 IR 条带在处理后 24 小时受到 NeBB 的强烈影响,表明氧化损伤和核酸降解效果较好。放疗后不久,肿瘤细胞对 NeBB 的敏感性似乎低于对 NeMBRT 的敏感性。尽管如此,一天后,NeBB 和高剂量 NeMBRT 区域都产生了重要的光谱修饰,表明细胞死亡过程、蛋白质氧化或氧化应激。 脂质相关光谱变化,特别是由于肿瘤细胞系的 NeBB 和 NeMBRT 峰组,与活性氧攻击一致。
更新日期:2024-12-13
中文翻译:

基于同步加速器的红外显微光谱揭示了健康和肿瘤细胞系对氖微束放射治疗的生物分子反应
使用当前的放疗 (RT) 技术管理放射耐药性肿瘤仍然很复杂。鉴于其有利的放射生物学特性,建议使用重离子束进行治疗。然而,先前对患者的研究对周围健康组织产生了严重的不利影响。因此,重粒子线 RT 可能受益于微束放射治疗 (MBRT) 的组织保留效应。为了研究这种组合的潜力,我们通过基于同步加速器的傅里叶变换红外显微光谱 (SR-FTIRM) 评估了对氖 MBRT (NeMBRT) 的生化反应。在 HIMAC 对健康 (BJ) 和肿瘤 (B16-F10) 细胞系进行无缝(宽光束)氖 RT (NeBB) 和 NeMBRT 检测。SR-FTIRM 测量在 ALBA 同步加速器的 MIRAS 光束线上进行。允许主成分分析 (PCA) 评估不同 RT 方式和剂量的照射后和照射后 24 小时的生化影响。对于健康细胞,NeMBRT 在照射后早期导致与未照射细胞最不同的光谱特征,主要是由于蛋白质构象修饰。尽管如此,大部分损伤似乎在 RT 后一天恢复了;相反,蛋白质和核酸相关的 IR 条带在处理后 24 小时受到 NeBB 的强烈影响,表明氧化损伤和核酸降解效果较好。放疗后不久,肿瘤细胞对 NeBB 的敏感性似乎低于对 NeMBRT 的敏感性。尽管如此,一天后,NeBB 和高剂量 NeMBRT 区域都产生了重要的光谱修饰,表明细胞死亡过程、蛋白质氧化或氧化应激。 脂质相关光谱变化,特别是由于肿瘤细胞系的 NeBB 和 NeMBRT 峰组,与活性氧攻击一致。































 京公网安备 11010802027423号
京公网安备 11010802027423号