当前位置:
X-MOL 学术
›
Pest Manag. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
New 5,6‐dihydrobenzo[h]quinoline derivatives as potential demethylase inhibitors (DMIs): design, synthesis, activity evaluation and molecular dynamics simulation
Pest Management Science ( IF 3.8 ) Pub Date : 2024-12-12 , DOI: 10.1002/ps.8594 Xiansong Xie, Jingwen Wang, Ailing Bao, Ziquan Deng, Deyuan Wang, Wenrui Chen, Wenjing Jiang, Weiyi Li, Xiaorong Tang, Yingkun Yan
Pest Management Science ( IF 3.8 ) Pub Date : 2024-12-12 , DOI: 10.1002/ps.8594 Xiansong Xie, Jingwen Wang, Ailing Bao, Ziquan Deng, Deyuan Wang, Wenrui Chen, Wenjing Jiang, Weiyi Li, Xiaorong Tang, Yingkun Yan
|
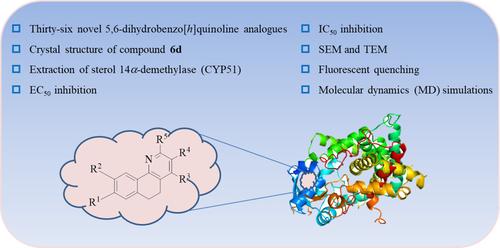
BACKGROUNDBipolaris maydis is a serious plant fungus and strongly affects the yield and quality of crops. The main control strategy is the employment of fungicides. To research efficient fungicide with novel structure, a series of novel 5,6‐dihydrobenzo[h ]quinoline derivatives were designed and synthesized.RESULTSThirty‐six novel 5,6‐dihydrobenzo[h ]quinoline analogues were designed and synthesized. The assay results showed that most compounds exhibited significant fungicidal activity against Pyricularia oryzae , Bipolaris maydis, Sclerotinia sclerotiorum , Penicillium digitatum and Valsa mali at 16 μg mL−1 . Compounds 4 h, 5e, 6a and 6b showed better antifungal activity than fluquinconazole against B . maydis . Their half maximal effective concentration (EC50 ) values were 0.732, 0.283, 0.529, 0.644 and 0.826 μg mL−1 , respectively. Furthermore, the bioactive compounds were determined against sterol 14α ‐demethylase (CYP51). The results indicated that they displayed prominent inhibiting activities, 4 h, 5e, 6a and 6b also had better inhibitory activities than fluquinconazole against CYP51. Their half maximal inhibitory concentration (IC50 ) values were 0.840, 0.315, 0.601, 0.750 and 1.018 μg mL−1 , respectively. The fluorescent quenching tests of proteins indicated that the quenching patterns of compounds 5e and 6a were analogous to fluquinconazole. The molecular dynamics (MD) simulations indicated that compound 5e possessed stronger affinity than fluquinconazole to CYP51.CONCLUSIONThe results of the present study displayed that novel 5,6‐dihydrobenzo[h ]quinoline derivatives could be one scaffold of potential CYP51 inhibitor and will provide some valuable information for the research and development of new fungicides. © 2024 Society of Chemical Industry.
中文翻译:

新的 5,6-二氢苯并 [h] 喹啉衍生物作为潜在的去甲基化酶抑制剂 (DMI):设计、合成、活性评价和分子动力学模拟
背景Bipolaris maydis 是一种严重的植物真菌,强烈影响农作物的产量和质量。主要的控制策略是使用杀菌剂。为了研究具有新颖结构的高效杀菌剂,设计并合成了一系列新型 5,6-二氢苯并[h]喹啉衍生物。结果设计并合成了 36 种新型 5,6-二氢苯并[h]喹啉类似物。测定结果表明,大多数化合物在 16 μg mL-1 时对米梨花、双极菌、菌核病菌、指青霉和苹果缬草表现出显著的杀真菌活性。化合物 4 h 、 5e 、 6a 和 6b 对 B. maydis 的抗真菌活性优于氟喹康唑。它们的半数最大有效浓度 (EC50) 值分别为 0.732、0.283、0.529、0.644 和 0.826 μg mL-1。此外,针对甾醇 14α-脱甲基酶 (CYP51) 测定生物活性化合物。结果表明,它们表现出突出的抑制活性,4 h 、 5e 、 6a 和 6b 对 CYP51 的抑制活性也优于氟喹康唑。它们的半数最大抑菌浓度 (IC50) 值分别为 0.840、0.315、0.601、0.750 和 1.018 μg mL-1。蛋白质的荧光淬灭试验表明,化合物 5e 和 6a 的淬灭模式类似于氟喹康唑。分子动力学 (MD) 模拟表明,化合物 5e 对 CYP51 的亲和力强于氟喹康唑。结论本研究结果表明,新型 5,6-二氢苯并[h]喹啉衍生物可能是潜在 CYP51 抑制剂的一种支架,将为新型杀菌剂的研发提供一些有价值的信息。© 2024 化工学会.
更新日期:2024-12-12
中文翻译:

新的 5,6-二氢苯并 [h] 喹啉衍生物作为潜在的去甲基化酶抑制剂 (DMI):设计、合成、活性评价和分子动力学模拟
背景Bipolaris maydis 是一种严重的植物真菌,强烈影响农作物的产量和质量。主要的控制策略是使用杀菌剂。为了研究具有新颖结构的高效杀菌剂,设计并合成了一系列新型 5,6-二氢苯并[h]喹啉衍生物。结果设计并合成了 36 种新型 5,6-二氢苯并[h]喹啉类似物。测定结果表明,大多数化合物在 16 μg mL-1 时对米梨花、双极菌、菌核病菌、指青霉和苹果缬草表现出显著的杀真菌活性。化合物 4 h 、 5e 、 6a 和 6b 对 B. maydis 的抗真菌活性优于氟喹康唑。它们的半数最大有效浓度 (EC50) 值分别为 0.732、0.283、0.529、0.644 和 0.826 μg mL-1。此外,针对甾醇 14α-脱甲基酶 (CYP51) 测定生物活性化合物。结果表明,它们表现出突出的抑制活性,4 h 、 5e 、 6a 和 6b 对 CYP51 的抑制活性也优于氟喹康唑。它们的半数最大抑菌浓度 (IC50) 值分别为 0.840、0.315、0.601、0.750 和 1.018 μg mL-1。蛋白质的荧光淬灭试验表明,化合物 5e 和 6a 的淬灭模式类似于氟喹康唑。分子动力学 (MD) 模拟表明,化合物 5e 对 CYP51 的亲和力强于氟喹康唑。结论本研究结果表明,新型 5,6-二氢苯并[h]喹啉衍生物可能是潜在 CYP51 抑制剂的一种支架,将为新型杀菌剂的研发提供一些有价值的信息。© 2024 化工学会.

































 京公网安备 11010802027423号
京公网安备 11010802027423号