当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
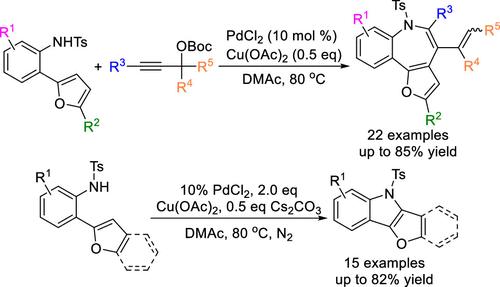
Controllable Synthesis of Benzo[b]furo[2,3-d]azepines or Furo[3,2-b]indoles via Intermolecular Oxidative Annulation of 2-(Furan-2-yl)anilines and Propargyl Carbonates versus Intramolecular C–H Amination Reactions
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-12-12 , DOI: 10.1021/acs.joc.4c02293 Rong Ma, Yujie Qu, Pengcheng Guan, Minghui Liu, Yu Han, Feng Feng, Chengyu Wang
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-12-12 , DOI: 10.1021/acs.joc.4c02293 Rong Ma, Yujie Qu, Pengcheng Guan, Minghui Liu, Yu Han, Feng Feng, Chengyu Wang
|
Two novel Pd-catalyzed protocols for the controllable synthesis of benzo[b]furo[2,3-d]azepines and furo[3,2-b]indoles have been developed by intermolecular oxidative annulation of 2-(furan-2-yl)anilines and propargyl carbonates versus intramolecular C–H amination reactions. These two protocols feature great scalability, functional group tolerance, and relatively mild reaction conditions. Notably, the robust methodologies could also provide valuable opportunities for assembling azepine-fused benzothiophene, indole-fused benzothiophene, and indole-fused benzimidazole, which may have potential applications in the synthesis of related pharmaceuticals or polymeric materials.
中文翻译:

通过 2-(呋喃-2-基)苯胺和丙炔基碳酸酯的分子间氧化环化可控合成苯并[b]呋喃[2,3-d]氮卓类药物或呋喃[3,2-b]吲哚与分子内 C-H 胺化反应
通过与分子内 C-H 胺化反应相比,2-(呋喃-2-基)苯胺和炔丙基碳酸酯的分子间氧化环化,开发了两种用于可控合成苯并[b]呋喃[2,3-d]氮卓类和呋喃[3,2-b]吲哚的新型方案。这两种方案具有很好的可扩展性、官能团耐受性和相对温和的反应条件。值得注意的是,稳健的方法还可以为组装氮卓类融合的苯并噻吩、吲哚稠融的苯并噻吩和吲哚融合的苯并咪唑提供宝贵的机会,这可能在相关药物或聚合物材料的合成中具有潜在应用。
更新日期:2024-12-12
中文翻译:

通过 2-(呋喃-2-基)苯胺和丙炔基碳酸酯的分子间氧化环化可控合成苯并[b]呋喃[2,3-d]氮卓类药物或呋喃[3,2-b]吲哚与分子内 C-H 胺化反应
通过与分子内 C-H 胺化反应相比,2-(呋喃-2-基)苯胺和炔丙基碳酸酯的分子间氧化环化,开发了两种用于可控合成苯并[b]呋喃[2,3-d]氮卓类和呋喃[3,2-b]吲哚的新型方案。这两种方案具有很好的可扩展性、官能团耐受性和相对温和的反应条件。值得注意的是,稳健的方法还可以为组装氮卓类融合的苯并噻吩、吲哚稠融的苯并噻吩和吲哚融合的苯并咪唑提供宝贵的机会,这可能在相关药物或聚合物材料的合成中具有潜在应用。






























 京公网安备 11010802027423号
京公网安备 11010802027423号