当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Robust Liquid Chromatography–Mass Spectrometry Determination of Deuterium Isotopologues for Quality Control of Deucravacitinib Using Nominal Mass Instrumentation
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-12-11 , DOI: 10.1021/acs.oprd.4c00361 Cong Bi, Yueer Shi, Wei Ding, James Chadwick, Yan Zha, Su Pan, Paul Foy, Nicola Hulme, Brent Kleintop
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-12-11 , DOI: 10.1021/acs.oprd.4c00361 Cong Bi, Yueer Shi, Wei Ding, James Chadwick, Yan Zha, Su Pan, Paul Foy, Nicola Hulme, Brent Kleintop
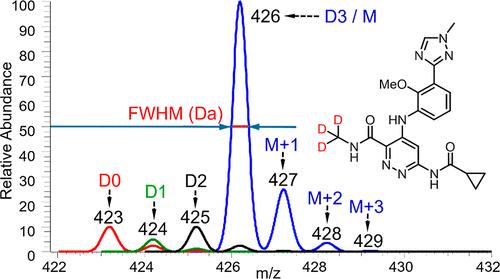
|
Deuterated drug molecules are a growing area of interest in the pharmaceutical industry, and controlling isotopologue impurities can be vital due to potential toxicity and efficacy concerns. The limited number of analytical approaches reported for this purpose often relies on high-end instrumentation that is not readily available in quality control (QC) laboratories. We developed and validated a robust liquid chromatography–mass spectrometry (LC–MS) method for the determination of isotopologue impurities in the deuterated drug SOTYKTU (deucravacitinib) using QC-friendly nominal mass LC–MS instruments. The method conditions were systematically evaluated and optimized to ensure key performance attributes, and the method was extended to assess isotopologue impurities in the input material d3-methylamine hydrochloride via an efficient derivatization procedure. The established conditions demonstrated excellent robustness and reproducibility, facilitating successful transfer from development laboratories to commercial QC laboratories. The successful implementation in the release testing of both input reagent and the final drug substance highlights the practical application of nominal mass LC–MS for the determination of the isotopic purity in pharmaceutical development.
中文翻译:

使用标称质量仪器对氘同位素体进行稳健液相色谱-质谱测定,用于 Deucravacitinib 的质量控制
氘代药物分子是制药行业日益关注的领域,由于潜在的毒性和疗效问题,控制同位素杂质可能至关重要。据报道,用于此目的的分析方法数量有限,通常依赖于质量控制 (QC) 实验室中不容易获得的高端仪器。我们开发并验证了一种稳定的液相色谱-质谱 (LC-MS) 方法,用于使用符合 QC 要求的标称质量 LC-MS 仪器测定氘代药物 SOTYKTU (deucravacitinib) 中的同位素杂质。系统地评估和优化了方法条件,以确保关键性能属性,并将该方法扩展为通过高效的衍生化程序评估输入材料 d3-甲胺盐酸盐中的同位素异构体杂质。既定条件表现出优异的稳定性和重现性,有助于从开发实验室成功转移到商业 QC 实验室。在输入试剂和最终原料药的放行测试中成功实施,凸显了标称质量 LC-MS 在药物开发中测定同位素纯度的实际应用。
更新日期:2024-12-11
中文翻译:

使用标称质量仪器对氘同位素体进行稳健液相色谱-质谱测定,用于 Deucravacitinib 的质量控制
氘代药物分子是制药行业日益关注的领域,由于潜在的毒性和疗效问题,控制同位素杂质可能至关重要。据报道,用于此目的的分析方法数量有限,通常依赖于质量控制 (QC) 实验室中不容易获得的高端仪器。我们开发并验证了一种稳定的液相色谱-质谱 (LC-MS) 方法,用于使用符合 QC 要求的标称质量 LC-MS 仪器测定氘代药物 SOTYKTU (deucravacitinib) 中的同位素杂质。系统地评估和优化了方法条件,以确保关键性能属性,并将该方法扩展为通过高效的衍生化程序评估输入材料 d3-甲胺盐酸盐中的同位素异构体杂质。既定条件表现出优异的稳定性和重现性,有助于从开发实验室成功转移到商业 QC 实验室。在输入试剂和最终原料药的放行测试中成功实施,凸显了标称质量 LC-MS 在药物开发中测定同位素纯度的实际应用。






























 京公网安备 11010802027423号
京公网安备 11010802027423号