当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective Synthesis of Chiral Acyclic Nitriles Containing α-All-Carbon Quaternary Stereocenters via Synergistic Palladium and Phase-Transfer Catalysis
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-12-11 , DOI: 10.1021/acscatal.4c06364 Cheng Guo, Yufei Dong, Yiyang Wang, Xuan Du, Runxia Ma, Choon-Hong Tan, Xinjun Luan, Jingyun Ren
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-12-11 , DOI: 10.1021/acscatal.4c06364 Cheng Guo, Yufei Dong, Yiyang Wang, Xuan Du, Runxia Ma, Choon-Hong Tan, Xinjun Luan, Jingyun Ren
|
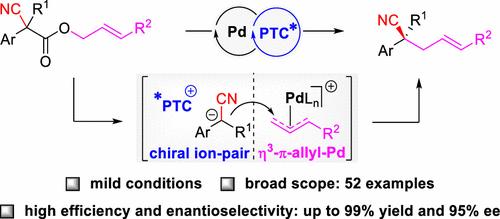
Herein, we present a practical strategy for the asymmetric synthesis of chiral acyclic nitriles featuring α-all-carbon quaternary stereocenters, utilizing synergistic palladium and phase-transfer catalysis from allyl 2-cyanoacetates under mild conditions. This approach offers an efficient and reliable method for the in situ generation of tertiary α-cyano carbanions through intramolecular palladium-catalyzed decarboxylative allylic alkylation. Additionally, it enables highly enantioselective control of simple nitriles via ion-pairing interactions with chiral phase-transfer catalysts. The synthetic utility of this method is further demonstrated by its scalability to gram-scale synthesis and its subsequent transformation into a variety of chiral functionalized compounds containing acyclic all-carbon quaternary stereocenters.
中文翻译:

通过协同钯和相转移催化合成含有 α-全碳四元立体中心的手性无环腈的对映选择性合成
在此,我们提出了一种不对称合成手性无环腈的实用策略,具有α全碳四元立体中心,在温和条件下利用来自烯丙基 2-氰基乙酸酯的协同钯和相转移催化。这种方法为通过分子内钯催化的脱羧烯丙基烷基化原位生成叔α-氰基碳负离子提供了一种高效可靠的方法。此外,它通过与手性相转移催化剂的离子对相互作用实现对简单腈的高度对映选择性控制。该方法的合成效用进一步证明了其对克级合成的可扩展性,以及随后转化为包含非环全碳四元立体中心的各种手性功能化化合物。
更新日期:2024-12-11
中文翻译:

通过协同钯和相转移催化合成含有 α-全碳四元立体中心的手性无环腈的对映选择性合成
在此,我们提出了一种不对称合成手性无环腈的实用策略,具有α全碳四元立体中心,在温和条件下利用来自烯丙基 2-氰基乙酸酯的协同钯和相转移催化。这种方法为通过分子内钯催化的脱羧烯丙基烷基化原位生成叔α-氰基碳负离子提供了一种高效可靠的方法。此外,它通过与手性相转移催化剂的离子对相互作用实现对简单腈的高度对映选择性控制。该方法的合成效用进一步证明了其对克级合成的可扩展性,以及随后转化为包含非环全碳四元立体中心的各种手性功能化化合物。






























 京公网安备 11010802027423号
京公网安备 11010802027423号