当前位置:
X-MOL 学术
›
Water Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Halogenated bisphenol F compounds: Chlorination-mediated formation and photochemical fate in sunlit surface water
Water Research ( IF 11.4 ) Pub Date : 2024-12-12 , DOI: 10.1016/j.watres.2024.122966 Shengqi Zhang, Yuefei Ji, Kyriakos Manoli, Yong Li, Qian Chen, Yunho Lee, Xin Yu, Mingbao Feng
Water Research ( IF 11.4 ) Pub Date : 2024-12-12 , DOI: 10.1016/j.watres.2024.122966 Shengqi Zhang, Yuefei Ji, Kyriakos Manoli, Yong Li, Qian Chen, Yunho Lee, Xin Yu, Mingbao Feng
|
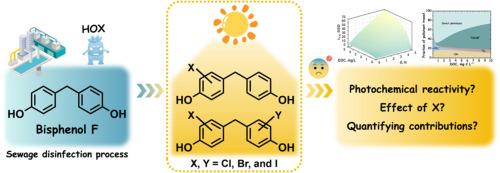
Halogenated bisphenol compounds are prevalent in urban water systems and may pose greater environmental risks than their bisphenol precursors. This study explored the formation of halogenated bisphenol F (BPF) in water chlorination and their subsequent transformation behaviors in receiving waters. The kinetics and pathways of BPF halogenation with chlorine, bromine, and iodine were firstly investigated. BPF chlorination followed second-order kinetics, with pH-dependent second-order rate constants (kapp) ranging from 1.0 M−1 s−1 at pH 5.0 to 50.4 M−1 s−1 at pH 9.0. The kapp of BPF with bromine and iodine were 4−5 orders of magnitude higher than those of chlorine. The degradation potential of halogenated BPF products in sunlit surface waters was also evaluated, focusing on both direct and indirect photolysis. Indirect photolysis, involving reactions with excited triplet state of CDOM (3CDOM*), •OH and 1O2, emerged as the primary degradation pathway for BPF, while both direct photolysis and indirect photolysis with 3CDOM* predominated for mono- and dihalogenated BPF products. Compared with BPF, the photodegradation of halogenated products was significantly enhanced. Photolysis experiments in wastewater-receiving wetland water demonstrated effective degradation of halogenated BPF products, highlighting the pivotal role of sunlight in their environmental fate. Overall, this study advances understanding of the formation and fate of halogenated BPF products and provides valuable insights for managing the environmental impacts of these emerging contaminants.
中文翻译:

卤代双酚 F 化合物:阳光照射的地表水中氯化介导的形成和光化学命运
卤代双酚化合物普遍存在于城市供水系统中,可能比其双酚前体构成更大的环境风险。本研究探讨了卤代双酚 F (BPF) 在水氯化中的形成及其在受纳水中随后的转化行为。首先研究了 BPF 与氯、溴和碘卤化的动力学和途径。BPF 氯化遵循二级动力学,pH 依赖性二级速率常数 (kapp) 范围从 pH 5.0 时的 1.0 M-1 s-1 到 pH 9.0 时的 50.4 M-1 s-1。BPF 与溴和碘的 kapp 比氯高 4-5 个数量级。还评估了卤化 BPF 产品在阳光照射的地表水中的潜在降解潜力,重点关注直接和间接光解。间接光解,包括与 CDOM 的激发三重态 (3CDOM*)、•OH 和 1O2 的反应,成为 BPF 的主要降解途径,而直接光解和间接光解与 3CDOM* 在单卤和二卤化 BPF 产物中占主导地位。与 BPF 相比,卤代产物的光降解性显著增强。在废水接收湿地水中进行的光解实验表明,卤化 BPF 产品可以有效降解,突出了阳光在其环境命运中的关键作用。总体而言,本研究促进了对卤代 BPF 产品的形成和归宿的理解,并为管理这些新兴污染物对环境的影响提供了有价值的见解。
更新日期:2024-12-12
中文翻译:

卤代双酚 F 化合物:阳光照射的地表水中氯化介导的形成和光化学命运
卤代双酚化合物普遍存在于城市供水系统中,可能比其双酚前体构成更大的环境风险。本研究探讨了卤代双酚 F (BPF) 在水氯化中的形成及其在受纳水中随后的转化行为。首先研究了 BPF 与氯、溴和碘卤化的动力学和途径。BPF 氯化遵循二级动力学,pH 依赖性二级速率常数 (kapp) 范围从 pH 5.0 时的 1.0 M-1 s-1 到 pH 9.0 时的 50.4 M-1 s-1。BPF 与溴和碘的 kapp 比氯高 4-5 个数量级。还评估了卤化 BPF 产品在阳光照射的地表水中的潜在降解潜力,重点关注直接和间接光解。间接光解,包括与 CDOM 的激发三重态 (3CDOM*)、•OH 和 1O2 的反应,成为 BPF 的主要降解途径,而直接光解和间接光解与 3CDOM* 在单卤和二卤化 BPF 产物中占主导地位。与 BPF 相比,卤代产物的光降解性显著增强。在废水接收湿地水中进行的光解实验表明,卤化 BPF 产品可以有效降解,突出了阳光在其环境命运中的关键作用。总体而言,本研究促进了对卤代 BPF 产品的形成和归宿的理解,并为管理这些新兴污染物对环境的影响提供了有价值的见解。






























 京公网安备 11010802027423号
京公网安备 11010802027423号