当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electronic structures of blue copper centers of amicyanin and azurin in the solution state
Dalton Transactions ( IF 3.5 ) Pub Date : 2024-12-12 , DOI: 10.1039/d4dt02891k Yudai Izumi, Ralph Ugalino, Jun Miyawaki, Chie Shibazaki, Motoyasu Adachi, Naoya Kurahashi, Hisao Kiuchi, Yoshihisa Harada, Kentaro Fujii
Dalton Transactions ( IF 3.5 ) Pub Date : 2024-12-12 , DOI: 10.1039/d4dt02891k Yudai Izumi, Ralph Ugalino, Jun Miyawaki, Chie Shibazaki, Motoyasu Adachi, Naoya Kurahashi, Hisao Kiuchi, Yoshihisa Harada, Kentaro Fujii
|
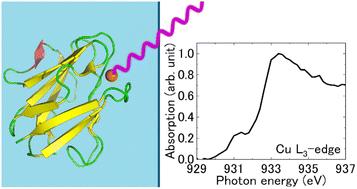
X-ray absorption near edge structure (XANES) spectra of blue copper proteins, amicyanin and azurin, in the solution state were measured in the copper L3-edge energy region. The absorption peak energies were quite similar for both proteins, while the main edge region for azurin was broader than that for amicyanin, owing to more pronounced shoulder spectral features in the former. Ab initio calculations at the whole protein level qualitatively reproduced the experimental spectra well. The relative X-ray absorption intensities suggest that the degree of covalency of the copper–ligand bond at the active site was weaker for amicyanin than that for azurin.
中文翻译:

溶液态下 amicyanin 和 azurin 的蓝铜中心的电子结构
在铜 L3 边缘能量区测量了蓝铜蛋白、酰胺花青素和天青素在溶液状态下的 X 射线吸收近边缘结构 (XANES) 光谱。两种蛋白质的吸收峰能量非常相似,而 azurin 的主要边缘区域比 amicyanin 更宽,因为前者具有更明显的肩光谱特征。全蛋白水平的 Ab 头计算定性地再现了实验光谱。相对 X 射线吸收强度表明,酰胺青素在活性位点的铜-配体键的共价程度比 Azurin 弱。
更新日期:2024-12-16
中文翻译:

溶液态下 amicyanin 和 azurin 的蓝铜中心的电子结构
在铜 L3 边缘能量区测量了蓝铜蛋白、酰胺花青素和天青素在溶液状态下的 X 射线吸收近边缘结构 (XANES) 光谱。两种蛋白质的吸收峰能量非常相似,而 azurin 的主要边缘区域比 amicyanin 更宽,因为前者具有更明显的肩光谱特征。全蛋白水平的 Ab 头计算定性地再现了实验光谱。相对 X 射线吸收强度表明,酰胺青素在活性位点的铜-配体键的共价程度比 Azurin 弱。






























 京公网安备 11010802027423号
京公网安备 11010802027423号